Question: Please help with part C /9/2022 11:59:00 PM End Date: 12/9/2022 11:59:00 PM (25%) Problem 3: A block of aluminum at a temperature of Ti
Please help with part C
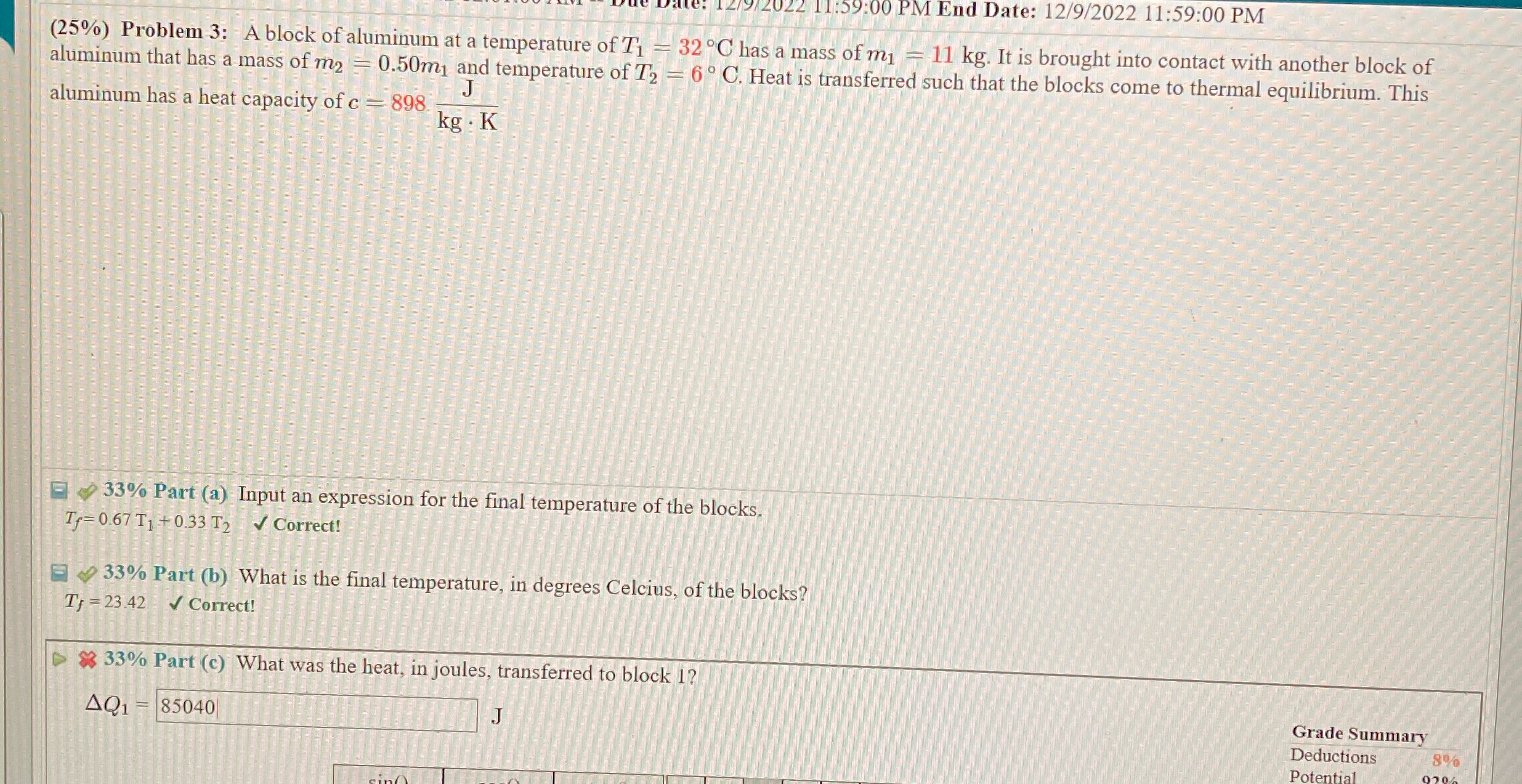
/9/2022 11:59:00 PM End Date: 12/9/2022 11:59:00 PM (25%) Problem 3: A block of aluminum at a temperature of Ti - 32 C has a mass of my = 11 kg. It is brought into contact with another block of aluminum that has a mass of m2 = 0.50m, and temperature of T2 = 6 C. Heat is transferred such that the blocks come to thermal equilibrium. This J aluminum has a heat capacity of c = 898 kg . K 33% Part (a) Input an expression for the final temperature of the blocks. If= 0.67 T1 + 0.33 T2 V Correct! 33% Part (b) What is the final temperature, in degrees Celcius, of the blocks? TJ =23.42 - Correct! $ 33% Part (c) What was the heat, in joules, transferred to block 1? AQ1 = 85040 Grade Summary Deductions 8% Potential
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


