Question: Please help with question 1 and 5 thank you Rates of Chemical Reactions I: A Clock Reaction To measare the effect of concentration upon the









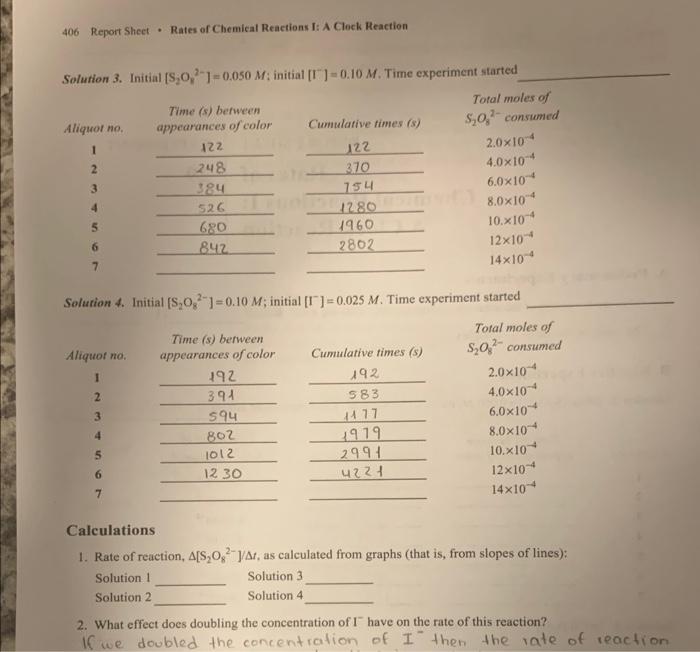

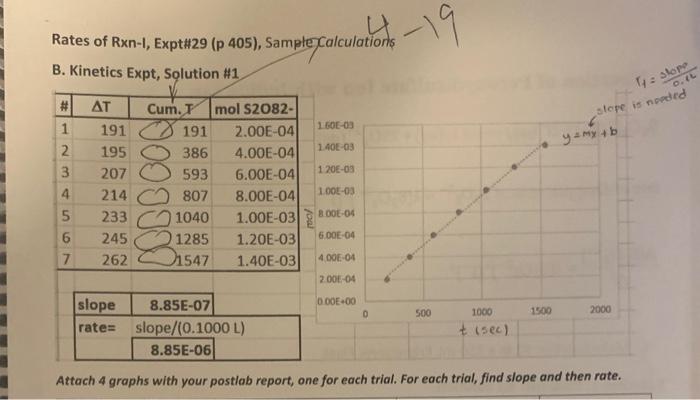
Rates of Chemical Reactions I: A Clock Reaction To measare the effect of concentration upon the rate of the reaction of peroxydisulfate ion with fodide ion; to deternine the order of the reaction with respect to the reactant concentrations; and to obtain the rute law for the chemical reaction. APPARATUS AND CHEMICALS WORK IN PAIRS, BUT EVALUATE YOUR DATA INDIVIDUALLY. Factors Affecting Rates of Reactions DISCUSSION On the basis of the experiments you've performed, you may have noticed that reactions occur at varying nites. There is an entire spectrum of nates of reactions, ranging from very slow to extremely fast. For example, the rusting of iron is reasonably slow, whereas the decomposition of TNT is extremely fast. The branch of chemistry concerned with the rates of reactions is called chemfical kinetics ( Section 14.1) Experiments show that rates of homogencous reactions in solution depend upon the following: 1. The nature of the reactants 2. The concentration of the reactints 3. The temperature 4. The presence of a eatalyst Before a reaction can occur, the reactants must come in direct contact via collisions of the reacting particles ( Section 14.5). However, even then, the reacting particles (ions or molecules) must collide with sufficient energy to result in a reaction. If they do not, their collisions are ineffective and analogous to collisions of biltiard balls. Kecping these considerations in mind, you can qualitatively explain how the various factors inflluence the rates of reactions. Copyright o201x Pearson Fducatiod, ine. Concentration Changing the concentration of a solution aliers the fumber of purticles per unit volume ( Section 14.3). The more particles present in a given volume, the greater the probubilisy of their colliding. Hence, increasing the concentration of a solution increases the number of collisions per unit time and therefore may increase the rate of reaction. Temperatare Because tempenture is a meauure of the avenge kinetic enerisy. an incruase in temperatire incrases the kinetic enerny of the particles ( Section 14.5). An increase in kinctic enerzy increases the velocity of the partcles and therefore the number of collisions betwoen them in a given period of time. Thus, the rate of reacticn increases. Also, an increase in kinetic energy results in a greater propontion of the collisions huving the required activition enengy for the reaction. As a rale of thamb, for each 10C increase in temperature, the rute of reaction doubles. Catalyst Catalysts, in some cases, are belicved to increase reaction rates by bringing particles into close juxtaposition in the correct geometrical armangement for reaction to oceur (e'Section 14.7). In other inctances, catalysts offer an aiternative route to the reaction, one that requires less energetic collisions between teactant particles. If less energy is required for a soceessful collision, a larer percentage of the collisions will have the reguisise energy, and the reaction will occur fister. Actually, the catalyst may ake an active part in the resction, but at the end of the reaction, the catalyst can be reconcrod chemically unchanged. Order of Reaction Defined Now examine precisely what is meant by the expression rafs of reaction ( Section 14.2). Consider the hypothetical reaction A+BC+D You can measure the rate of this rextion by obierving the rate of disappeafance of either of the reactunts A and B ar the rate of appearines of either of the prodocts C and D. In practice, then, you measarn the chanso of concentration with time of A, B, C, or D. Which pocies you choose to observe is a matter of convenience. For cumple, if A,B, and D ate coloriess and C is colored, you could conveniently measure the rate of appeinnee of C by observing an increate in the intenicty of the color of the soltitica as a function of time. Mathematically, the rate of reaction may be expressed as follows: nteofdiappearanceofA=ingesequiredforchangechangeinonecretrationofA=tAA]niteofappeananceofC=tinerequirdforchangturseincoseentrationofC=i(C) In general, the rate of the reaction will depend upon the concentrutions of the feactants. Thus the rate of the bypotbetical reaction may be expressed as nate=k{S}[B} where [A] and [B] are the molar concentritions of A and B,x and y are the pou-: en to which the respective coesentraiaes mat be raiked to deseribe the nite, and k is the specific nate combuet Ooe of the objectites of chemical kinetics is to detemmine the rate law. Stated slichtly differently, one goal of measuring the rate of the reaction is to deteruine the numencal valucs of x and y in Equation [2]. Suppose you found that x=2 and y=1 for this sesction. Then rrite=M{A]2[B] Wotld be the rate law, It shonld be cvident froct Fquation [3] that doulhting the concentration of B (lecoping [A] the saine) wosild case the reaction rate to dotbie, On the other hand, doubling the sonceatration of A (kocping (B) the same) would cause the rate to increase by a factor of 4 bocatre the fate of the reaction is proportional to the square of the concentration of A. The powers to which the concentrations in the rate law are raised are termed the oraler of the rencelon. ( Section 14.3). In this case, the reaction is sald to be sccond order in A and fint order in. B. The ovenall orsler of the reaction is the sum of the esponents. 2+1=3,08a thirt-order reaction ( Section 14.3). It is possible to determine the order of the reaction by noting the effects of changing reagent cencentrations on the rate of the reaction. Note that the order of a reaction may be cand frequentiy in) difforent froen the stoichiometry of the reaction. Keep in mind that k, the specific rate constant, has a definite value that is independent of the concentration. It is characterintic of a giver reaction and depends upon temperature oelly. Once that rate law and the rate are known, the value of k can be calculated. Reaction of Peroxydisulfate Ion with lodide Ion In this experiment, you will meanure the rate of the reaction 52O42(aq)+212(aq)I2(aq)+25042(aq) and you will determine the rate law by moasuring the amount of peroxydinulfate, S2O32. What reacts as a function of time. The rate las to be determined is of the form bate of disappearace of S2O32+=k2S22Ir[I2]2 or (S2O82+1=A2S2O22)(t2)2 Your goal will be to determine the values of x and y as well as the specific rate constant, k You will add to the molution a small amount of another reagent (sodium thiosulfate, Nu2S2O3 ), which will cause a change in the color of the solution. The amount is such that the eolor change will occur when 2104 mol of S2O62 has reacted. For reasons to be explained shortly, the solution will turn blue-black when 2104 mol of S2O62 has reacted, You will quiekly add another portion of Nh2S2O3 after the appearance of the color, and the blueblack color will disappear. When the blue-black color reappears the second time, another 2104 mot of S2O2 has reacted, making a total of 2(2104). mol of S2O12 that has reacted. You will repeat this procedure several times, keeping careful note of the time for the appearance of the blue-black colors. By graphing the amount of S2O42 coniumed verain time, you will be able to deternine the rate of the reaction. By changing the initial concentrations of S2O52 and I and observing the effects upon the rate of the reaction, you will defermine the order of the reactige with retpect to S2O42 and I. The blue-black color that appears in the reaction is due to the presence of a starch-iodine complex that is formed from iodine. I1. and starch in the solution. Therefore, the color will not appotr until a detectable amount of t2 is forned accotding to Equation [4]. The thionalfate that is added to the soletion reacts extremely rapidilv with the iodine, as follows: I2(aq)+2S2O32(aq)21(aq)+S4O62(aq) Conscqueatly, until all of the S2O22 - that is added is consumed, there will not be a sufficient amount of t2 in the solution to yleld the bluc-black color. You will add 4104 mol of S2O32 each time (these equal portions are termed afiquoss). From the stoichiometry of Equations [4] and [6], you can verify that when this quantity of S2O32 has reacted, 2104 mol of S2O22 has reacted. Note also that althoogh iodide, 1, is consumed according to Equation [4], it is rapidly regenerited according to Equation [6]; therefore, its concentration docs not clange during a given experiment. Graphical Determination of Rate The more rapidly the 2104 mol of S2O42 is consumed, the faster the reaction. To determine the rate of the reaction, a plot of moles of S2O82 that have reacted versus the time required for the resction is made, as shown in Figure 29.1. The bost atraight line passing through the origin is drawn, and the slope is determined. The slope, AS2O82/Av, corresponds to the moles of S2O82 that have been consumed per second and is proportional to the rate. Because the rate corresponds to the change in the concentration of S2Oh. per second, dividing the slope by the volume of the solution yields the rate of disappearance of S2Oj2 (that is, A2S2O22l/Ar). If the total volume of the solution in this example was 75mL, the rate would be as follows: 0.075L4.5105mol/s=6.0104molLs If you obtain a rate of 6.0104mol/L-s when [S2O82]=2.0M and [1]=2.0M and a rate of 3.0104molLk when [S2O2]=1.0Mf and [I] ] 2.0M, you know that doubling the concentration of S2O52 doubles the rate of the reaction and the reaction is first order in S2O42. By varying the initial concentrations of S2O42 and I2, you can, via the above type of analysis, determine the order of the reaction with respect to both species. Ledvrahare iypvrimiatr AFigufe 29.1 Grephical aletermination of rote. GIVEIT SOME THOUGHT What does the meepness of the slope tell you about the rate of the reaction? Helpful Comments L. According to the procedure of this experiment, the solution will turn blue-black when exacely 2104 mol of S2O32 has ruacted. 2. The purpose of the KNO3 solution in this revction is to keep the neatetion medraw the same in each run in terms of the coacentration of ions; it does not enter into the reaction in any way. 3. The reaction studied in this experimen is catalyzed by metal ions. Theparpose of the drop of the EDTA ialution is to minimize the effects of trace quantities of metal ion impurities that woold cause spurious effects on the reaction: 4. You will perform a few preliminary experiments to become acquainted with the obiervaticas in thte experificent so that you will know what to expect in the reactions. 5. The initial concentrations of the reactants have been peovided on the report sheel. 2. Repeat the procedure in (1), but when the solution changes colof add four atropis of a4N.NNaS2O3. mit the solution, and twote the cffot that the addition of Na2S2O3 has on the color. B. Kineties Experiment Equipment Setup Set up two burets held by a clamp on a ring stand as shown in Figure 29.2. Une these burets to accurately measure the volumes of the KI and KNO3 solutions. Use two separate 1mL pipets for meavuring the volumes of the Na2S2O3 and sarch solutions and we 25mL and 50mL pipets to meksure. the volumes of the (NH4)2S2O4 solutions. Solation Preparation Prepare four resction solutions as follows (prepare the nest solution only when you have completely finishod with the previous one): Each solution must be frechly prepared before you to begin the rate stadythat is, prepane solutions 1. 2, 3, and t one at a time ar you make your meas. wnements. GIVE IT SOME THOUGHT Why is a drop of EDTA added to cach solution? Rate Measurements Prepare solution 1 in a 250mL Erlenmeyer flusk that has been scrupulously cleaned and dried. Pipet 25.0mL of (NH4h2S2O4 solution into a clean, dry 100mL beaker. Be ready to begin timing the reaction when the solutions are mixed (READ AHEAD). The reaction starts the moment the solations are mixed. BE PREPAREDI ZERO TBME! Quickly pour the 25.0mL of (NH+)2S2O4 solution into solution 1 and swirl vigotously, note to the nearest second the time you begin mixing At the instant the blus-black color appeary, 2104mol of S2O42 has reacted, intentiatebl' (be peparedl) add a 1mL atiquot of Na2S2O3 solution from the pipet and swirl the solution; the color will dissppeer, By filling each of seven clean, dry test rubes with 1mL of Nu2S2O2 solution, to avoid losing time, you can add these aliquots to your reaction. when the blue color appears. Record the time for the reappearance of the blue-black color. Add another 1mL aliquot of Na2S2O3 solution and note the time for the resppearance of the color. The time interval being measured is that between appearances of the blue-black color For good results, these aliquots of N4S2O3. must be. measured as quiekly, accurately, and reproducibly us possible. Continue this procedure until you have added seven aliquots to solution 1. GIVE IT SOME THOUGHT What does the blue-blacic color indicate? You are finished with solution I when you have recorded all of your times on the report shect. (The rime intervale are cumblotive.) Solutions 2, 3, and 4 should be beated in the same manner except that 50.0mL portions of (NH4)2S2O2 solations should be added to solations 2 and 4 and 25mL of (NH4)2S2O4 solution sheald be added to sotution 3.(CAUTION : Be on gaiand solution 2 will react more rapidly than solution t.) in each of these reactions, the firial total solution volume is exactly 100mL. Calculations Use the data sheet to thbulate the following for each aliquot of Na1S2O3 added to cach of the four solutions: 1. The time intetval from the stant of the reaction (additioa of S2Ox2 ) to the appearance of color for the first aliquot of S2O32 and the time interval from the preceding color appearance for cach succeeting atiquot (column 2). Cosrright 92010 Rearsas Idsuadurt, the. GIVE IT SOME THOUGHT a. Do you expect your graph to be linear or exponential? b. What does this tell you about the overall order? Waste-Disposal Instructions No wastes from this experiment should be flushed down the sink. Locate the special containers placed in the laboratory for the disposal of excess iodide and peroxydisulfate solutions as well as for the reaction mixtures from the test tubes or flasks. All wastes should be disposed of in these containers. Rate of reaction drepend of , molavity (M=Lmal) - temp (keep it constant) r=rL[S2Oe]x[I]y A. Preliminary Experiments s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


