Question: Please help with question 11 at the top and question 12 at the bottom! 11. Consider ethyl acetate. a. which protons are expected to be
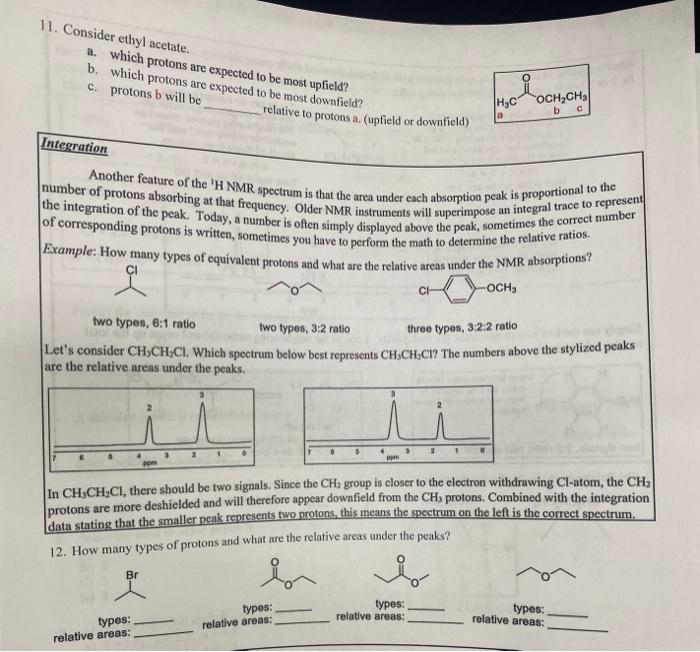
11. Consider ethyl acetate. a. which protons are expected to be most upfield? b. which protons are expected to be most downfield? c. protons b will be relative to protons a. (upfield or downfield) Integration Another feature of the ' H NMR spectrum is that the area under each absorption peak is proportional to the number of protons absorbing at that frequency. Older NMR instruments will superimpose an integral trace to represent the integration of the peak. Today, a number is often simply displayed above the peak, sometimes the correct number of corresponding protons is written, sometimes you have to perform the math to determine the relative ratios. Example: How many types of equivalent protons and what are the relative areas under the NMR absorptions? two types, 6:1 ratio two types, 3:2 ratio three types, 3:2:2 ratio Let's consider CH3CH2Cl. Which spectrum below best represents CH3CH2Cl ? The numbers above the stylized peaks are the relative areas under the peaks. In CH3CH2Cl, there should be two signals. Since the CH2 group is closer to the electron withdrawing Cl atom, the CH3 : protons are more deshielded and will therefore appear downfield from the CH3 protons. Combined with the integration data stating that the smaller peak represents two protons, this means the spectrum on the left is the correct spectrum. 12. How many types of protons and what are the relative areas under the peaks? types: relative areas: types: relative arees: types
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


