Question: Please help with question 2. Please write in a clear and legible manner. Thank you. Week #3 Homework Propagation of Error and Comparison of Means
Please help with question 2. Please write in a clear and legible manner. Thank you.
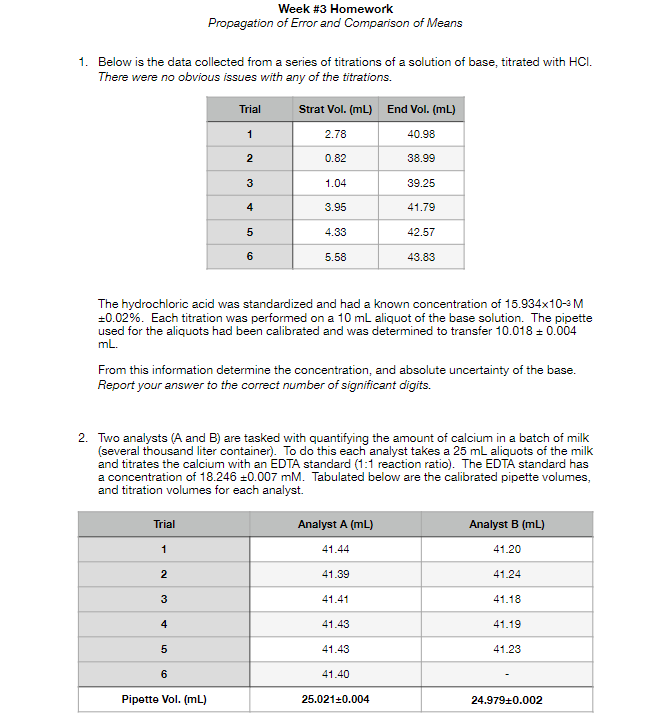
Week #3 Homework Propagation of Error and Comparison of Means 1. Below is the data collected from a series of titrations of a solution of base, titrated with HCI. There were no obvious issues with any of the titrations. Trial Strat Vol. (mL) End Vol. (mL) 2.78 40.98 1 2 0.82 38.99 3 1.04 39.25 4 4 3.95 41.79 5 4.33 42.57 6 5.58 43.83 The hydrochloric acid was standardized and had a known concentration of 15.934x10-4M +0.02%. Each titration was performed on a 10 mL aliquot of the base solution. The pipette used for the aliquots had been calibrated and was determined to transfer 10.018 +0.004 mL. From this information determine the concentration, and absolute uncertainty of the base. Report your answer to the correct number of significant digits. 2. Two analysts (A and B) are tasked with quantifying the amount of calcium in a batch of milk (several thousand liter container). To do this each analyst takes a 25 mL aliquots of the milk and titrates the calcium with an EDTA standard (1:1 reaction ratio). The EDTA standard has a concentration of 18.246 +0.007 mM. Tabulated below are the calibrated pipette volumes, and titration volumes for each analyst. Trial Analyst A (mL) Analyst B (mL) 41.20 1 41.44 2 41.39 41.24 3 41.41 41.18 4 41.43 41.19 5 41.43 41.23 6 41.40 Pipette Vol. (mL) 25.021+0.004 24.979+0.002
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


