Question: please help with question #9 and 10 Objective: Determine the relationship between the pressure and volume of a confined gas. Theory: ideal gas law In

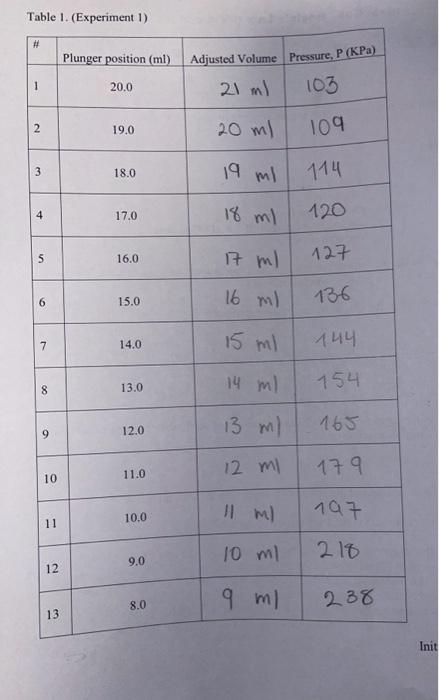
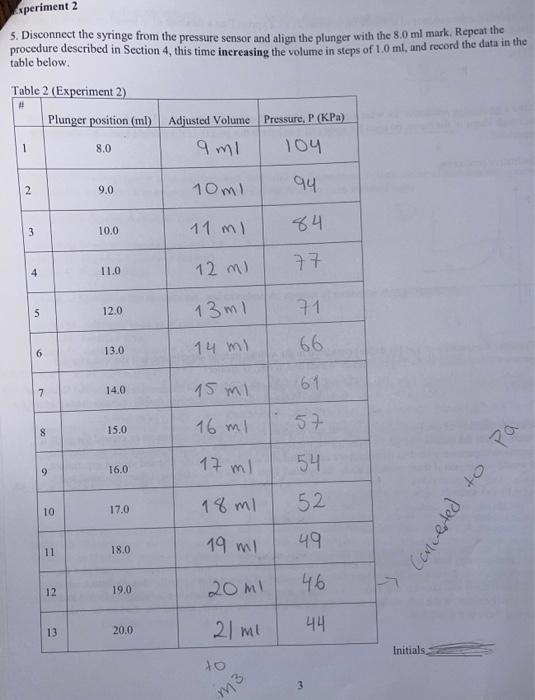

Objective: Determine the relationship between the pressure and volume of a confined gas. Theory: ideal gas law In this experiment, you will measure the pressure of confined gas as a function of changing volume, fit your data to verify the ideal gas law, and analyze possible sources of error. 1. Record the temperature and pressure in the lab on the day of the experiment: T=22.5C=295.5KP=1020HPaH=102KPa 2. Turn on the SPARK interface unit, plug in the PASCO Pressure Sensor and verify that the sensor reads the atmospheric pressure before the syringe is attached. Experiment 1 3. Align the stopper (always use the outer edge) of the syringe's plunger with the 20.0ml mark. 4. Connect the syringe and the pressure sensor via the 1cm piece of plastic tubing. Record the pressure reading when the stopper is aligned with the 20.0ml mark in the first line of the table below, then change the volume in steps of 1.0ml, wait for the pressure reading to stabilize before recording it for each volume listed in the table. The reading on the syringe has a systematic error because of the extra volume of the plastic tube and assembly that connects the syringe to the pressure sensor (outlined on the picture below). We will account for this by estimating that the extra volume is approximately 1.0ml. Fill in the second column on the next page by adding 1.0ml to each position reading in order to obtain the Adjusted Volume. Note: because the extra volume that we are adding is an estimate, the values in the second column will be less precise (although likely more accurate). Be mindful that each lab partner completes at least one table. Table 1. (Experiment 1) 5. Disconnect the syringe from the pressure sensor and align the plunger with the 8.0ml mark. Repeat the procedure described in Section 4 , this time inereasing the volume in steps of 1.0ml, and record the data in the table below. 9. Plot the following data in a spreadsheet (such as Excel) for Experiment 1: the number of moles in the system as it changes from one measurement to the next; and the P(V) curve in standard units. Type in the following data from Table 1: Column A: Number of measurement (1,2,3,.12,13) Column B: Adjusted Volume of gas (ml) Column C: Pressure (KPa) Convert to SI units in the spreadsheet, and calculete the number of moles for each measurement: Column D: Adjusted Volume of gas (m3) Column E: Pressure (Pa) Column F: number of moles n=PV/RT (ideal gas law: the values for R and T are on the 1st page; P and V should be in SI units) Insert two plots in the same sheet: (a) bar chart showing the number of moles in the system as a function of the measurement number; (b) scatter chart for P(V) in standard (SI) units. Fit the P(V) plot as a power function: (a function of the form y=ax ). What do you expect will be the values of a and b in this function? (Hint: see Section 6 where you derived the formula.) Take the value for the number of moles n from your experimental data (e.g. initial value at 20ml ) For the ideal gas, expected values: b= a= derive formula for a (a) Right click on any data point on the plot, select "Add trend line" from the menu. (b) Select "Power" from the choice of functions in the dialog box. (c) Click on "Options" tab in the dialog box, select "Display equation on chart". 10. On the second sheet in the same spreadsheet file, enter the data for the Experiment 2 and repeat all the steps listed in Section 9. Again, take the value of n from experiment. Expected values: b= a=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


