Question: please help with questions 2-4. Part III: Computations (WebMO) - Complete the table below. Table 3: Structural parameters determined using computational chemistry model. MOLECULE HYBRIDIZATION*
 please help with questions 2-4.
please help with questions 2-4.
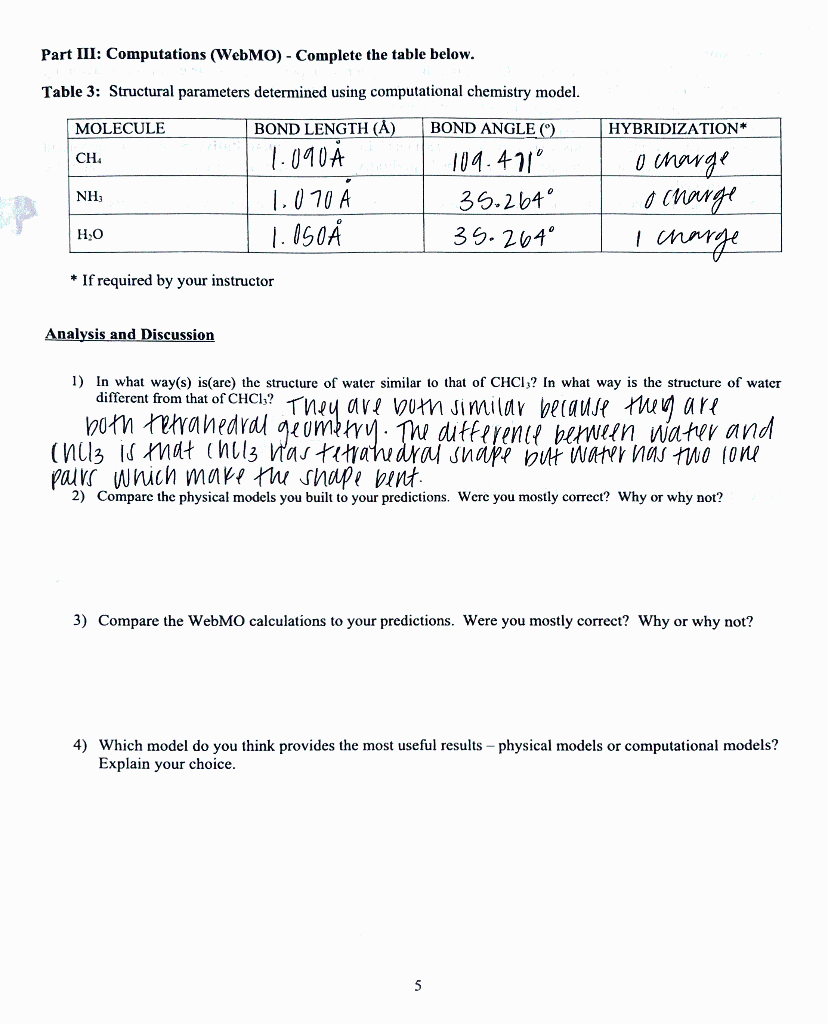
Part III: Computations (WebMO) - Complete the table below. Table 3: Structural parameters determined using computational chemistry model. MOLECULE HYBRIDIZATION* BOND LENGTH (A) 1.090 CH BOND ANGLE (9) 109.471 35.264 35.264 NH 1.070 A o charge o charge I charge H.O 1.050 * If required by your instructor Analysis and Discussion 1) In what way(s) isare) the structure of water similar to that of CHCI;? In what way is the structure of water different from that of CHCI.? They are both similar because they are both hava nedral geometry. The autference permeten water and (nils is that incls was te trahedral snape but water has two lone pairs which make the shape bent. 2) Compare the physical models you built to your predictions. Were you mostly correct? Why or why not? 3) Compare the WebMO calculations to your predictions. Were you mostly correct? Why or why not? 4) Which model do you think provides the most useful results - physical models or computational models? Explain your choice. 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


