Question: please help with table 3 and show all work, also if you can please put the final answers in table for so its easy to
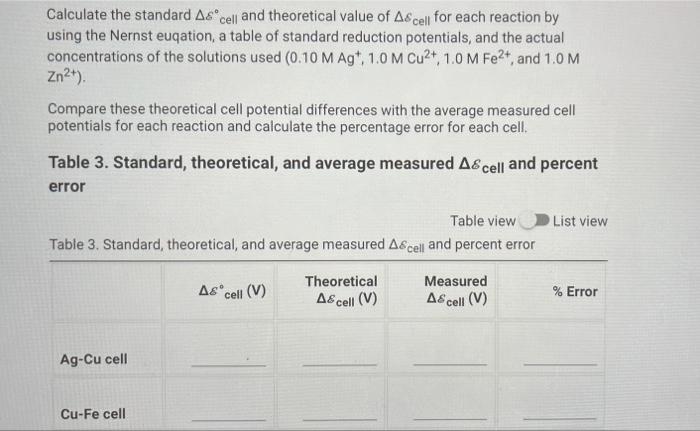

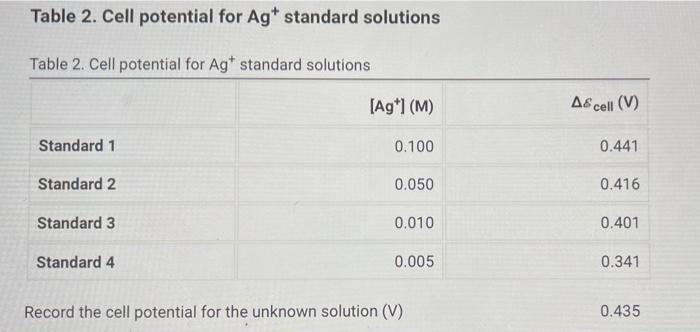
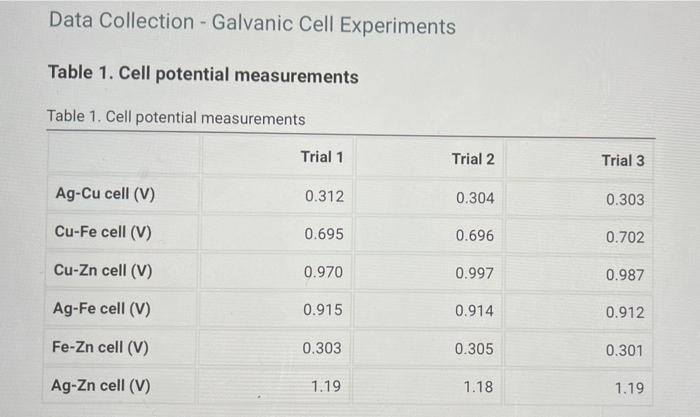
Calculate the standard Accell and theoretical value of Accell for each reaction by using the Nernst eucation, a table of standard reduction potentials, and the actual concentrations of the solutions used (0.10 M Agt, 1.0 M Cu2+, 1,0 M Fe2+, and 1.0 M Zn2+). Compare these theoretical cell potential differences with the average measured cell potentials for each reaction and calculate the percentage error for each cell. Table 3. Standard, theoretical, and average measured A& cell and percent error List view Table view Table 3. Standard, theoretical, and average measured A& cell and percent error Ascell (V) Theoretical A8cell (V) Measured A& cell (V) % Error Ag-Cu cell Cu-Fe cell Cu-Zn cell Ag-Fe cell Fe-Zn cell Ag-Zn cell Table 2. Cell potential for Agt standard solutions Table 2. Cell potential for Agt standard solutions [Ag+1(M) A8cell (V) Standard 1 0.100 0.441 Standard 2 0.050 0.416 Standard 3 0.010 0.401 Standard 4 0.005 0.341 Record the cell potential for the unknown solution (V) 0.435 Data Collection - Galvanic Cell Experiments Table 1. Cell potential measurements Table 1. Cell potential measurements Trial 1 Trial 2 Trial 3 Ag-Cu cell (V) 0.312 0.304 0.303 Cu-Fe cell (V) 0.695 0.696 0.702 Cu-Zn cell (V) 0.970 0.997 0.987 Ag-Fe cell (V) 0.915 0.914 0.912 Fe-Zn cell (V) 0.303 0.305 0.301 Ag-Zn cell (V) 1.19 1.18 1.19
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


