Question: please help with the calculations portion. I don't think I got the Unknown number correctly I do not understand by take more time in the
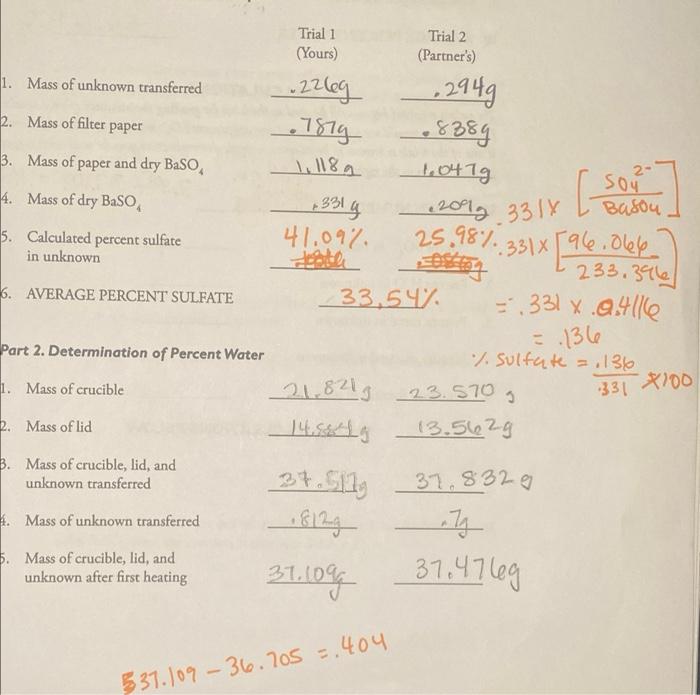


Trial 1 (Yours) Trial 2 (Partner's) 1. Mass of unknown transferred P 2. Mass of filter paper 3. Mass of paper and dry Baso 2- 4. Mass of dry BaSO .22leg 2949 1514- .8389 1.118a 1.047g sou +331 g 2012 3314 Basou 41.09% 25.98%. 331 [ace. dele Betong : 233.3966 33.54 = .331 x.2.4116 [ 5. Calculated percent sulfate in unknown 6. AVERAGE PERCENT SULFATE =.136 Part 2. Determination of Percent Water % sulfate = .136 1. Mass of crucible 21,821 23.570 g 2. Mass of lid 13.562g 331 *100 b. Mass of crucible, lid, and unknown transferred H. Mass of unknown transferred 5. Mass of crucible, lid, and unknown after first heating 37.S11 El ang 31.109 37.8329 ng 37.47leg 3 537.109 - 36.705 =.404 Name 6. Mass of crucible, lid, and unknown after second heating 37.474 7. Mass of unknown after heating 8. Mass of water lost 37. 109g 404 36.3429 +3589 50.2% 51.4. 50.127 408 9. Calculated percent water 10. AVERAGE PERCENT WATER CALCULATIONS The values reported below will be used as the basis for grading the accuracy of your results. Normally, you would use your calculated average percent of and percent H,O. However, if one of the trials is known to be in error, a single determination may be used with a note of explanation to the right. -367057 37,109 = 404 UNKNOWN NUMBER: 404 gm 1. Percent SO-in unknown 2. Percent H,O in unknown 3. Percent metal in unknown 4. Identity of metal in unknown 5. Empirical formula of unknown SHOW CALCULATIONS BELOW Name 6. Mass of crucible, Bd, and unknown after second heating 31. 109g 404 7. Mass of unknown after hearing 31.474 36342y 3589 5147. 8. Mass of water lost 408 50.2% 9. Calculated percent water 10. AVERAGE PERCENT WATER /.50 CALCULATIONS The walues reported below will be msed as the basis for grading the annuncy of your results. Normally you would use your calculated and percent So, and percent 7,0. However, if one of the trials is known to be in error a single determination may be used with note ef explanation to the night -367057 37.109 = 404 UNKNOWN NUMBER: .404gn 1. Percent Soin unknown 2. Percent H,O in unknown 3. Percent metal in unknown 4. Identity of metal in unknown 5. Empirical formula of unknown SHOW CALCULATIONS BELOW
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


