Question: Please help with the first and second question. Please make sure it is right. Thanks. For the second question please emphasize the units. Rate Law
Please help with the first and second question. Please make sure it is right. Thanks. For the second question please emphasize the units.
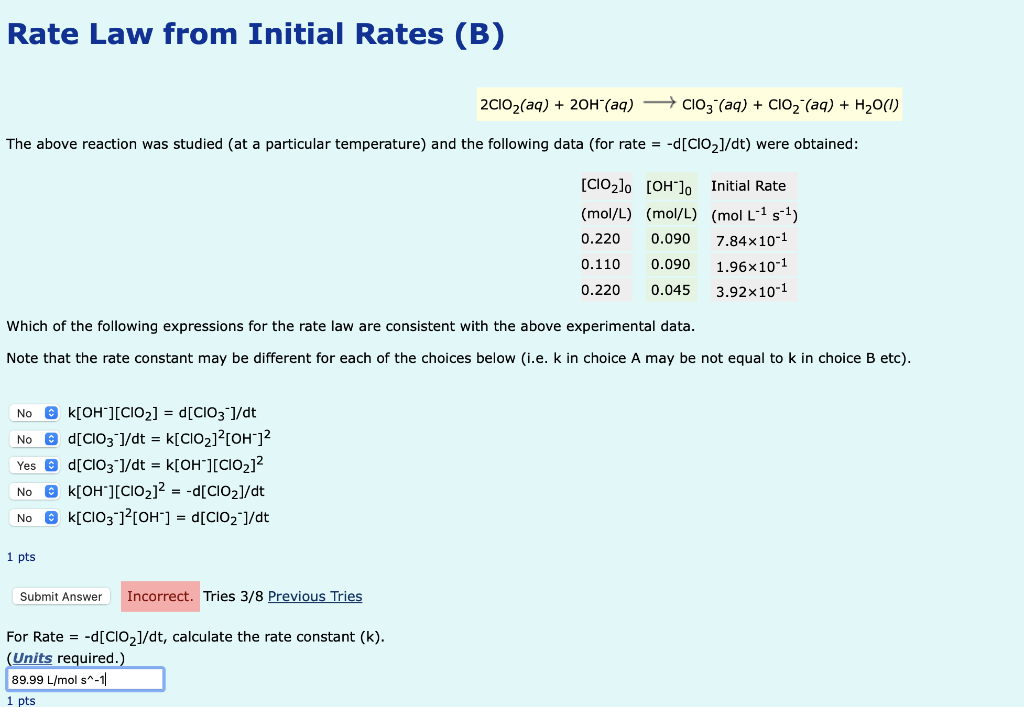
Rate Law from Initial Rates (B) 2ClO2(aq)+2OH(aq)ClO3(aq)+ClO2(aq)+H2O(l) The above reaction was studied (at a particular temperature) and the following data (for rate =d[ClO2]/dt ) were obtained: Which of the following expressions for the rate law are consistent with the above experimental data. Note that the rate constant may be different for each of the choices below (i.e. k in choice A may be not equal to k in choice B etc). k[OH][ClO2]=d[ClO3]/dtd[ClO3]/dt=k[ClO2]2[OH]2d[ClO3]/dt=k[OH][ClO2]2k[OH][ClO2]2=d[ClO2]/dtk[ClO3]2[OH]=d[ClO2]/dt 1 pts For Rate =d[ClO2]/dt, calculate the rate constant (k)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


