Question: please help with the followong question a. Briefly explain how a sample should be prepared for analysis by 'H NMR spectroscopy. In your answer account
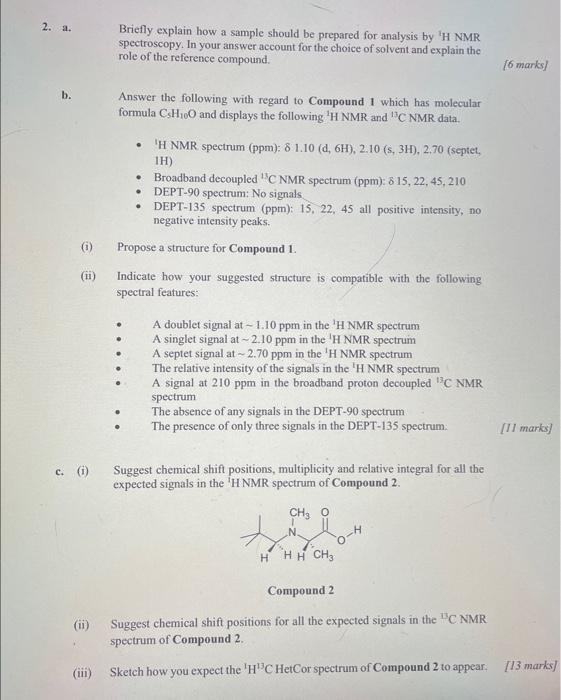
a. Briefly explain how a sample should be prepared for analysis by 'H NMR spectroscopy. In your answer account for the choice of solvent and explain the role of the reference compound. b. Answer the following with regard to Compound 1 which has molecular formula C5H10O and displays the following 1HNMR and 13C NMR data. - 'H NMR spectrum (ppm): 1.10(d,6H),2.10(s,3H),2.70 (septet, IH) - Broadband decoupled 13C NMR spectrum (ppm): 615,22,45,210 - DEPT-90 spectrum: No signals - DEPT-135 spectrum (ppm): 15, 22, 45 all positive intensity, no negative intensity peaks. (i) Propose a structure for Compound 1. (ii) Indicate how your suggested structure is compatible with the following spectral features: - A doublet signal at 1.10ppm in the 3H NMR spectrum - A singlet signal at 2.10ppm in the H NMR spectrum - A septet signal at 2.70ppm in the 1H NMR spectrum - The relative intensity of the signals in the 'H NMR spectrum - A signal at 210ppm in the broadband proton decoupled 13C NMR spectrum - The absence of any signals in the DEPT-90 spectrum - The presence of only three signals in the DEPT- 135 spectrum. c. (i) Suggest chemical shift positions, multiplicity and relative integral for all the expected signals in the 'H NMR spectrum of Compound 2. Compound 2 (ii) Suggest chemical shift positions for all the expected signals in the 10C NMR spectrum of Compound 2. (iii) Sketch how you expect the 1H13C HetCor spectrum of Compound 2 to appear
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


