Question: please help with the table Calculations for Table 2 on p. 174 1. Calculate molar concentrations of copper for each of brass solutions, brass), by

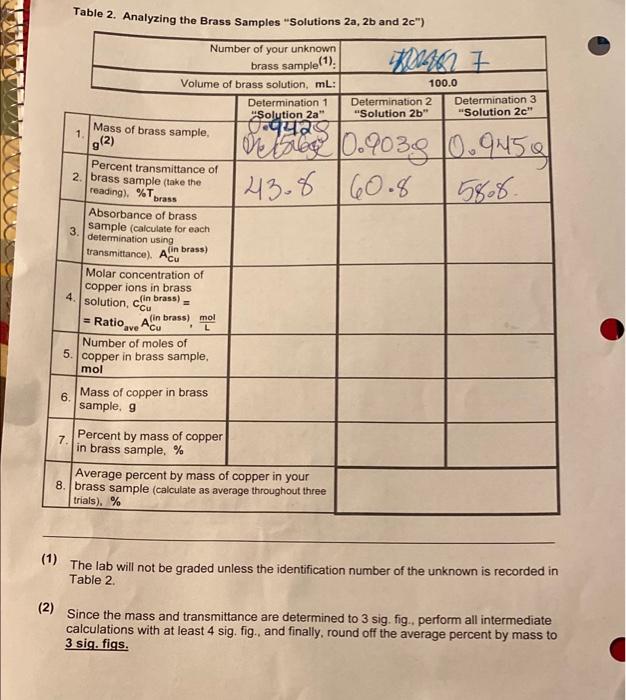
Calculations for Table 2 on p. 174 1. Calculate molar concentrations of copper for each of brass solutions, brass), by Cu multiplying the average ratio from Table 1 by the corresponding absorbances of a brass solutions: cln brass) - Ratio ACB (in brass) Perform further calculations for number of moles and mass of copper in your brass samples, using volumes of solutions (100 mL) and molar mass of copper 2. Calculate percent, by mass, of copper in each of brass samples for each of the solutions: %Cu = mass of copper in a brass sample 100% total mass of brass sample 3. Calculate the average percent by mass in 3 trials and answer the post lab. questions. Table 2. Analyzing the Brass Samples Solutions 2a, 2b and 2c") WW462 7 100.0 Determination 2 Determination 3 "Solution 2b" "Solution 2c." | Oct 0.9039 0.9M50 143.8 60.8 945g 58.8. Number of your unknown brass sample(1) Volume of brass solution, ml: Determination 1 "Solution 2a" Mass of brass sample gequa 1 9(2) Percent transmittance of 2. brass sample take the reading). %T brass Absorbance of brass sample (calculate for each 3. determination using transmittance). Acu fin brass) Molar concentration of copper ions in brass 4 solution, co brass) (in L Number of moles of copper in brass sample, mol - Ratio.ve Ado brass), mol . 5. 6. Mass of copper in brass sample, 9 7 Percent by mass of copper in brass sample. % Average percent by mass of copper in your 8. brass sample (calculate as average throughout three trials). % (1) The lab will not be graded unless the identification number of the unknown is recorded in Table 2. (2) Since the mass and transmittance are determined to 3 sig. fig., perform all intermediate calculations with at least 4 sig. fig., and finally, round off the average percent by mass to 3 sig. figs
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


