Question: please help You prepare Reaction mixture C following Table 2 in the Experimental section of the lab manual, using 2.00mL0.168MKI. What is the initial I


 please help
please help
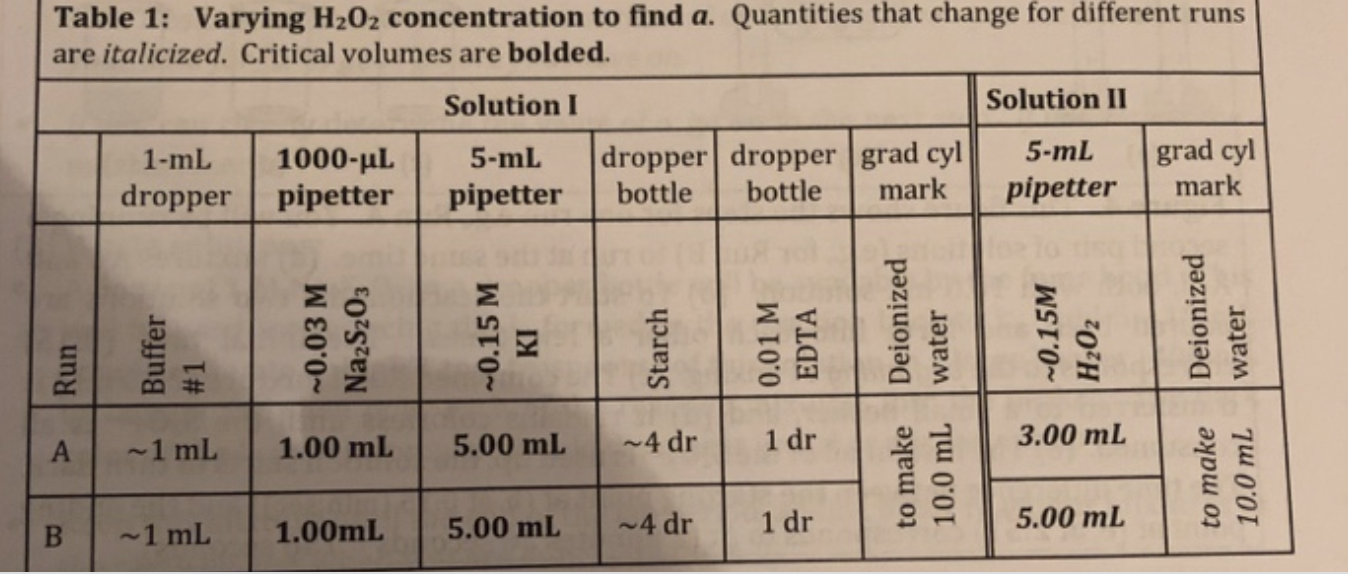
You prepare Reaction mixture C following Table 2 in the Experimental section of the lab manual, using 2.00mL0.168MKI. What is the initial I - molar concentration in the reaction mixture? Enter the numerical value to 3 significant figures, without units. To be counted as correct, your answer should be within 1% of the properly calculated value. If you maintain 3 significant figures in your calculations, your answer should satisfy this requirement without any problems. Enter your answer in standard decimal format (e.g:, 0.0123), NOT in scientific notation. A reaction is carried out with [H2O2]=0.000791M, with a reaction time of 2 minutes 57 seconds. What is the reaction rate, with units of M/s ? Enter the numerical value in scientific notation to 3 significant figures. Use "E-notation" to enter values in scientific notation , e.g., 1.23104 would be entered as 1.23E4 (no spaces, capital E). To be counted as correct, you answer should be within 1% of the correctly calculated value. Table 1: Varying H2O2 concentration to find a. Quantities that change for different runs are italicized. Critical volumes are bolded. You carry out Run A based on Table 1 in the lab manual, using 0.0147MNa2S2O3. What is [H2O2 ] (in molar units)? Enter the numerical value, without units, to 3 significant figures. To be counted as correct, your answer must be within 1% of the properly calculated value, so maintain 3 significant figures in your calculations. Also, the sign of the answer is important. Enter your answer in standard decimal format (e.g:, 0.0123), NOT in scientific notation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


