Question: please help!2006 change other liter 1 1 4. ( 25 ) A+ R; R + R k=1 like moulmine ,,2 - Train, 95%5%R,10% A90%R,C =

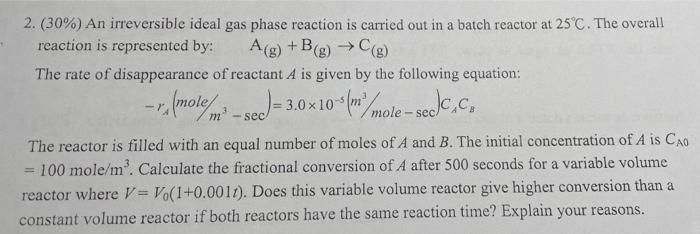
liter 1 1 4. ( 25 ) A+ R; R + R k=1 like moulmine ,,2 - Train, 95%5%R,10% A90%R,C = Cao + CRO - CA + Ce=41010/iter (a)CA,10-TA),CA , (b)(a)? (C) PER CSTR,(a)(), PER CSTR space time [Hint: dx 1 ( 2 + by b 1 = - In ax + b 2. (30%) An irreversible ideal gas phase reaction is carried out in a batch reactor at 25C. The overall reaction is represented by: A(g) +B(g) () The rate of disappearance of reactant A is given by the following equation: --,(molem m-sed) = 3 - -3.0x10() mole-sede.ch The reactor is filled with an equal number of moles of A and B. The initial concentration of A is Cao - 100 mole/m. Calculate the fractional conversion of A after 500 seconds for a variable volume reactor where V = Vo(1+0.0011). Does this variable volume reactor give higher conversion than a constant volume reactor if both reactors have the same reaction time? Explain your reasons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


