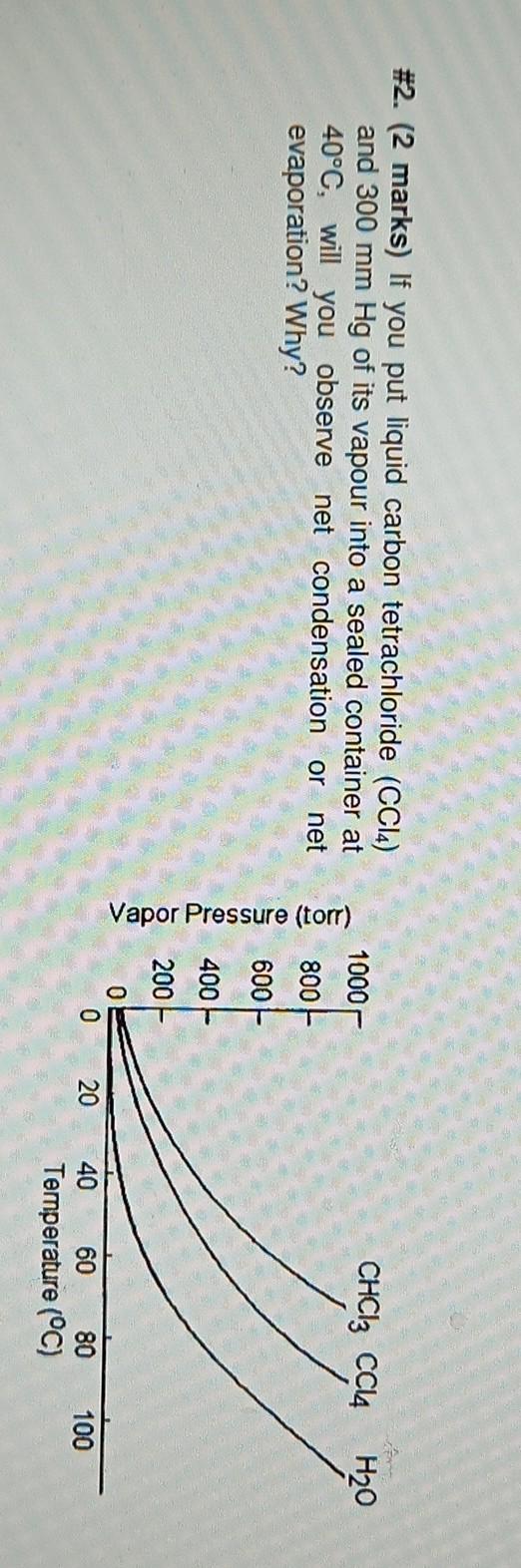
Question: please helppp #2. (2 marks) If you put liquid carbon tetrachloride (CCl4) and 300 mm Hg of its vapour into a sealed container at 40C,
please helppp
#2. (2 marks) If you put liquid carbon tetrachloride (CCl4) and 300 mm Hg of its vapour into a sealed container at 40C, will you observe net condensation or net evaporation? Why? 1000 CHCl3 CCl4 H20 800 600 Vapor Pressure (tor) LLLLL 400 200 0 20 40 100 60 80 Temperature (C)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


