Question: please highlight your final answer, thank you. Question 32 6 pts Using the following bond energies: Bond Bond Energy (kJ/mol) C=C 839 C-H 413 O=0
please highlight your final answer, thank you.
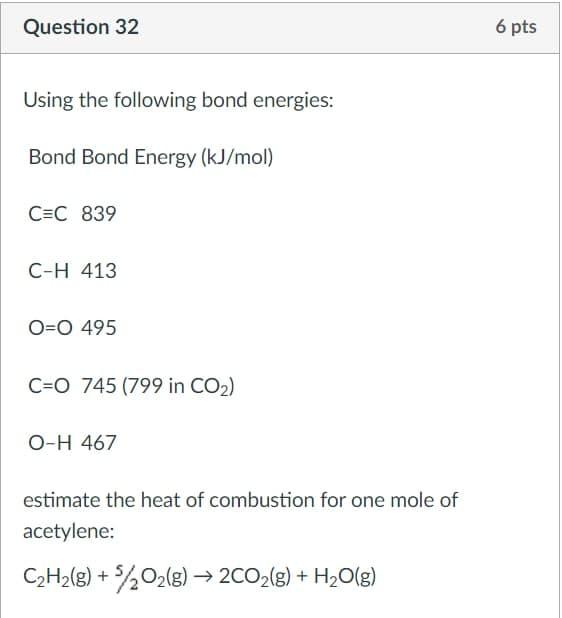
Question 32 6 pts Using the following bond energies: Bond Bond Energy (kJ/mol) C=C 839 C-H 413 O=0 495 C=0 745 (799 in CO2) O-H 467 estimate the heat of combustion for one mole of acetylene: C2H2(g) + 5,02(g) 2CO2(g) + H2O(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


