Question: please how would you do the second column(Br) and theoretical yield? BROMINATION OF ACETANILIDE AND ANILINE Pre La Questions 1. Fill in the appropriate data
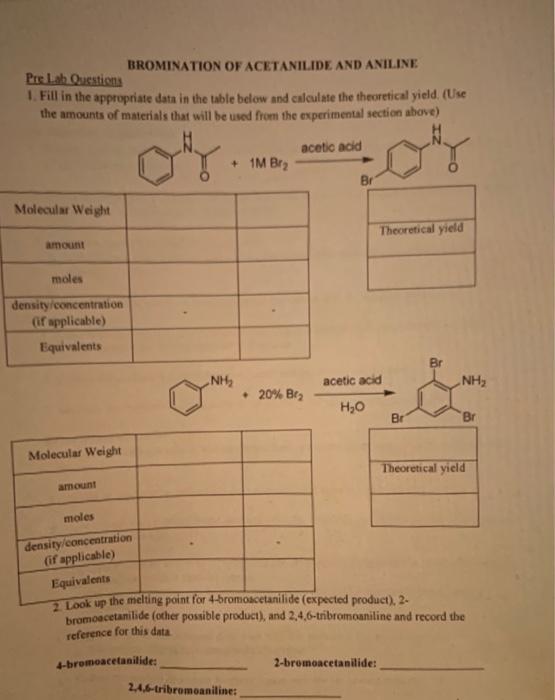

BROMINATION OF ACETANILIDE AND ANILINE Pre La Questions 1. Fill in the appropriate data in the table below and calculate the theoretical yield. (Use the amounts of materials that will be used from the experimental section above) acetic acid + 1M Bry Br Molecular Weight Theoretical yield amount moles density concentration (if applicable) Equivalents Br NH acetic acid NH, 20% Bez ,0 Br Br Molecular Weight Theoretical yield amount males density concentration (if applicable) Equivalents 2 Look up the melting point for 4-bromoacetanilide (expected product), 2- bromoacetanilide (other possible product), and 2,4,6-tribromoaniline and record the reference for this data -bromacetanilide: 2-bromoacetanilide: 2.4.6-tribromoaniline: Bromination of Acetanilide 1. Support a small test tube in an Erlenmeyer flask and to it add 0.07 g of acetanilide and 0.2 ml of concentrated acetic acid 2. Shake the tube gently until the solids are dissolved and add drop wise 1.0 M bromine in acetic acid until the solution remains orange in color 3. Allow the reaction to stand for 10 minutes. a. If the orange color lightens, add a few more drops of bromine to ensure bromine is in excess 4. After 10 minutes, add 2 ml water and swirl the tube to mix the solution thoroughly 5. Add NaHSO, drop wise (swirl often to mix the solution) to reduce the excess bromine a. The orange color should completely disappear 6. Collect the product in a small Buchner funnel and dry for several minutes 7. Measure the mass and melting point of the product and calculate the percent yield. Bromination of Aniline 1. Support a small test tube in an Erlenmeyer flask, place the setup on a balance, and zero the balance 2. While on the balance, add aniline drop wise until the mass is approximately 0.05 g 3. Add 0 2 ml concentrated acetic acid and swirl the tube until the aniline is fully dissolved or dispersed in the acid. 4. Dilute the solution with 0.5 mL water and add drop wise 20% bromine in acetic acid until the entire reaction mixture remains orange. The reaction mixture can be stirred with a glass stir rod (or wooden stick) to ensure complete mixing 5. Add 2 ml water and then add drop wise NaHSO, to reduce the excess bromine. a. The orange color should completely disappear 6. Collect the product in a small Buchner funnel and dry for several minutes. 7. Measure the mass and melting point of the product and calculate the percent yield
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


