Question: PLEASE I NEED HELP ASAP!! pleaseee Communication Part: 15 Marks An Investigation is carried out in which evidence is collected for the hypothetical reaction: W
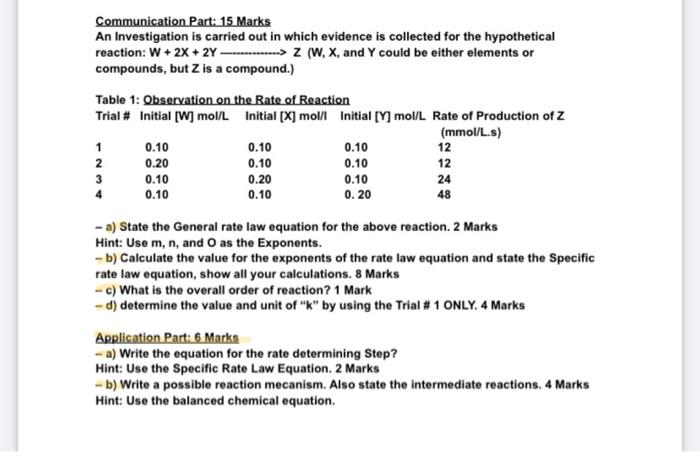
Communication Part: 15 Marks An Investigation is carried out in which evidence is collected for the hypothetical reaction: W + 2X + 2Y -------------> Z (W, X, and Y could be either elements or compounds, but Z is a compound.) Table 1: Observation on the Rate of Reaction Trial # Initial [W] mol/L Initial [X] mol/l Initial [Y] mol/L Rate of Production of Z (mmol/L.s) 1 0.10 0.10 0.10 12 2 0.20 0.10 0.10 12 3 0.10 0.20 0.10 24 4 0.10 0.10 0.20 48 - a) State the General rate law equation for the above reaction. 2 Marks Hint: Use m, n, and as the Exponents. - b) Calculate the value for the exponents of the rate law equation and state the Specific rate law equation, show all your calculations. 8 Marks - c) What is the overall order of reaction? 1 Mark - d) determine the value and unit of "k" by using the Trial # 1 ONLY. 4 Marks Application Part:6 Marks - a) Write the equation for the rate determining Step? Hint: Use the Specific Rate Law Equation. 2 Marks - b) Write a possible reaction mecanism. Also state the intermediate reactions. 4 Marks Hint: Use the balanced chemical equation. Communication Part: 15 Marks An Investigation is carried out in which evidence is collected for the hypothetical reaction: W + 2X + 2Y -------------> Z (W, X, and Y could be either elements or compounds, but Z is a compound.) Table 1: Observation on the Rate of Reaction Trial # Initial [W] mol/L Initial [X] mol/l Initial [Y] mol/L Rate of Production of Z (mmol/L.s) 1 0.10 0.10 0.10 12 2 0.20 0.10 0.10 12 3 0.10 0.20 0.10 24 4 0.10 0.10 0.20 48 - a) State the General rate law equation for the above reaction. 2 Marks Hint: Use m, n, and as the Exponents. - b) Calculate the value for the exponents of the rate law equation and state the Specific rate law equation, show all your calculations. 8 Marks - c) What is the overall order of reaction? 1 Mark - d) determine the value and unit of "k" by using the Trial # 1 ONLY. 4 Marks Application Part:6 Marks - a) Write the equation for the rate determining Step? Hint: Use the Specific Rate Law Equation. 2 Marks - b) Write a possible reaction mecanism. Also state the intermediate reactions. 4 Marks Hint: Use the balanced chemical equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


