Question: PLEASE I NEED HELP HOMEWORK 11 MATLAB PROGRAMMING EXERCISE: COMPARTMENT MODEL Remember: You must write MATLAB code and then execute your code to produce output

PLEASE I NEED HELP
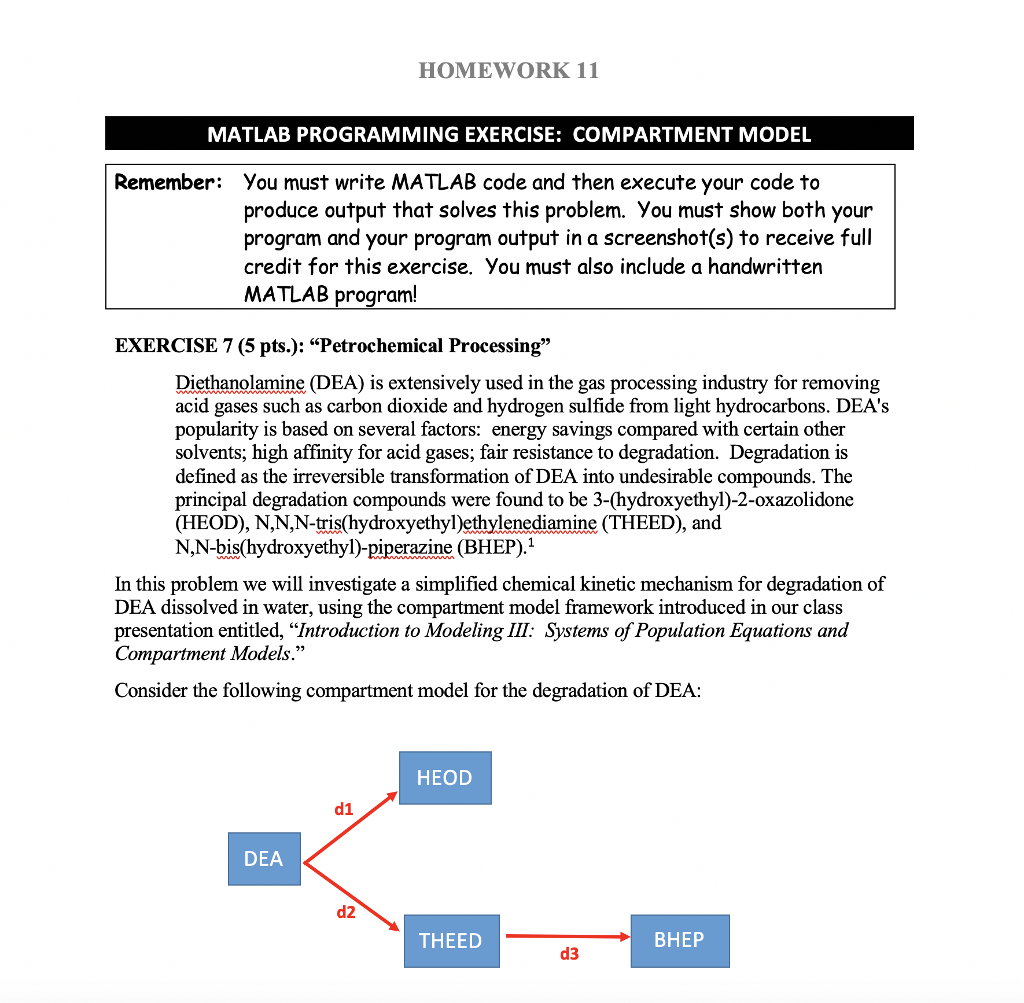
HOMEWORK 11 MATLAB PROGRAMMING EXERCISE: COMPARTMENT MODEL Remember: You must write MATLAB code and then execute your code to produce output that solves this problem. You must show both your program and your program output in a screenshot(s) to receive full credit for this exercise. You must also include a handwritten MATLAB program! EXERCISE 7 (5 pts.): "Petrochemical Processing" Diethanolamine (DEA) is extensively used in the gas processing industry for removing acid gases such as carbon dioxide and hydrogen sulfide from light hydrocarbons. DEA's popularity is based on several factors: energy savings compared with certain other solvents; high affinity for acid gases; fair resistance to degradation. Degradation is defined as the irreversible transformation of DEA into undesirable compounds. The principal degradation compounds were found to be 3-(hydroxyethyl)-2-oxazolidone (HEOD), N,N,N-tris(hydroxyethyl)ethylenediamine (THEED), and N,N-bis(hydroxyethyl)-piperazine (BHEP).1 In this problem we will investigate a simplified chemical kinetic mechanism for degradation of DEA dissolved in water, using the compartment model framework introduced in our class presentation entitled, "Introduction to Modeling III: Systems of Population Equations and Compartment Models." Consider the following compartment model for the degradation of DEA: HEOD d1 DEA d2 THEED d3 DEA dissociates (degrades) into HEOD at a rate of 0.0167% per minute, as indicated by the di pathway in the compartment diagram. DEA also dissociates into THEED at a rate of 0.025% per minute, as indicated by the d2 pathway. Finally, THEED degrades into BHEP at a rate of 0.0633% per minute, as indicated by the d3 pathway Kennard, ML and Meisen, A., "Mechanisms and Kinetics of Distanolamne Degradation", Industrial & Engineering Chemistry Fundamentals, 1985, 24, 129-140 bk2 Page 3 of 5 HOMEWORK 11 Note: In Chemistry the term "mass fraction" is commonly used to refer to the fraction of a solution that is a given chemical. So saying "a 0.65 mass fraction" means that within the solution, 65% is the given chemical. A 0.0 mlass fraction means that the chemical is NOTpresent. This term is used so that chemists do not have to worry about the volume or quantity of the whole solution Questions: In the initial solution, DEA's mass fraction is 0.65, and the initial solution mass fractions of HEOD, THEED and BHEP all equal 0.0. (Don't worry about the other 0.35 mass fraction, it is not important in this problem.) Compute (and display in the Command Window) what mass fractions are of DEA, HEOD, THEED and BHEP at the end of 33 hours? [Hint: 33 hours can be written as 33*60 minutes to keep the units all the same./ Compute (and display in the Command Window) the 4 individual mass fractions at the end of 100 hours? Prepare a plot of the variation over time of the individual mass fractions of DEA HEOD, THEED and BHEP, from 1 minute (i.e., initial concentration at the end of the first minute) to 7,200 minutes Verify (and display) that the total mass fraction of the combination of DEA, HEOD, THEED and BHEP remains at 0.65 after 7,200 minutes 1. 2. 3. 4. NOTE: DEA simultaneously dissociates into two different products, and thus, it "dies twice" so to speak. The overall "death rate" for DEA is therefore the sum of its two, individual dissociation rates. HOWEVER, both HEOD and THEED receive immigrants from DEA at rates corresponding to the rate (dl or d2) that points into their respective compartments! HOMEWORK 11 MATLAB PROGRAMMING EXERCISE: COMPARTMENT MODEL Remember: You must write MATLAB code and then execute your code to produce output that solves this problem. You must show both your program and your program output in a screenshot(s) to receive full credit for this exercise. You must also include a handwritten MATLAB program! EXERCISE 7 (5 pts.): "Petrochemical Processing" Diethanolamine (DEA) is extensively used in the gas processing industry for removing acid gases such as carbon dioxide and hydrogen sulfide from light hydrocarbons. DEA's popularity is based on several factors: energy savings compared with certain other solvents; high affinity for acid gases; fair resistance to degradation. Degradation is defined as the irreversible transformation of DEA into undesirable compounds. The principal degradation compounds were found to be 3-(hydroxyethyl)-2-oxazolidone (HEOD), N,N,N-tris(hydroxyethyl)ethylenediamine (THEED), and N,N-bis(hydroxyethyl)-piperazine (BHEP).1 In this problem we will investigate a simplified chemical kinetic mechanism for degradation of DEA dissolved in water, using the compartment model framework introduced in our class presentation entitled, "Introduction to Modeling III: Systems of Population Equations and Compartment Models." Consider the following compartment model for the degradation of DEA: HEOD d1 DEA d2 THEED d3 DEA dissociates (degrades) into HEOD at a rate of 0.0167% per minute, as indicated by the di pathway in the compartment diagram. DEA also dissociates into THEED at a rate of 0.025% per minute, as indicated by the d2 pathway. Finally, THEED degrades into BHEP at a rate of 0.0633% per minute, as indicated by the d3 pathway Kennard, ML and Meisen, A., "Mechanisms and Kinetics of Distanolamne Degradation", Industrial & Engineering Chemistry Fundamentals, 1985, 24, 129-140 bk2 Page 3 of 5 HOMEWORK 11 Note: In Chemistry the term "mass fraction" is commonly used to refer to the fraction of a solution that is a given chemical. So saying "a 0.65 mass fraction" means that within the solution, 65% is the given chemical. A 0.0 mlass fraction means that the chemical is NOTpresent. This term is used so that chemists do not have to worry about the volume or quantity of the whole solution Questions: In the initial solution, DEA's mass fraction is 0.65, and the initial solution mass fractions of HEOD, THEED and BHEP all equal 0.0. (Don't worry about the other 0.35 mass fraction, it is not important in this problem.) Compute (and display in the Command Window) what mass fractions are of DEA, HEOD, THEED and BHEP at the end of 33 hours? [Hint: 33 hours can be written as 33*60 minutes to keep the units all the same./ Compute (and display in the Command Window) the 4 individual mass fractions at the end of 100 hours? Prepare a plot of the variation over time of the individual mass fractions of DEA HEOD, THEED and BHEP, from 1 minute (i.e., initial concentration at the end of the first minute) to 7,200 minutes Verify (and display) that the total mass fraction of the combination of DEA, HEOD, THEED and BHEP remains at 0.65 after 7,200 minutes 1. 2. 3. 4. NOTE: DEA simultaneously dissociates into two different products, and thus, it "dies twice" so to speak. The overall "death rate" for DEA is therefore the sum of its two, individual dissociation rates. HOWEVER, both HEOD and THEED receive immigrants from DEA at rates corresponding to the rate (dl or d2) that points into their respective compartments
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


