Question: please I need help with question 1, 2 and 3, if you can not answer all of them please answer at least 2 and 3.
please I need help with question 1, 2 and 3, if you can not answer all of them please answer at least 2 and 3. thank you, I'll give you thumbs up.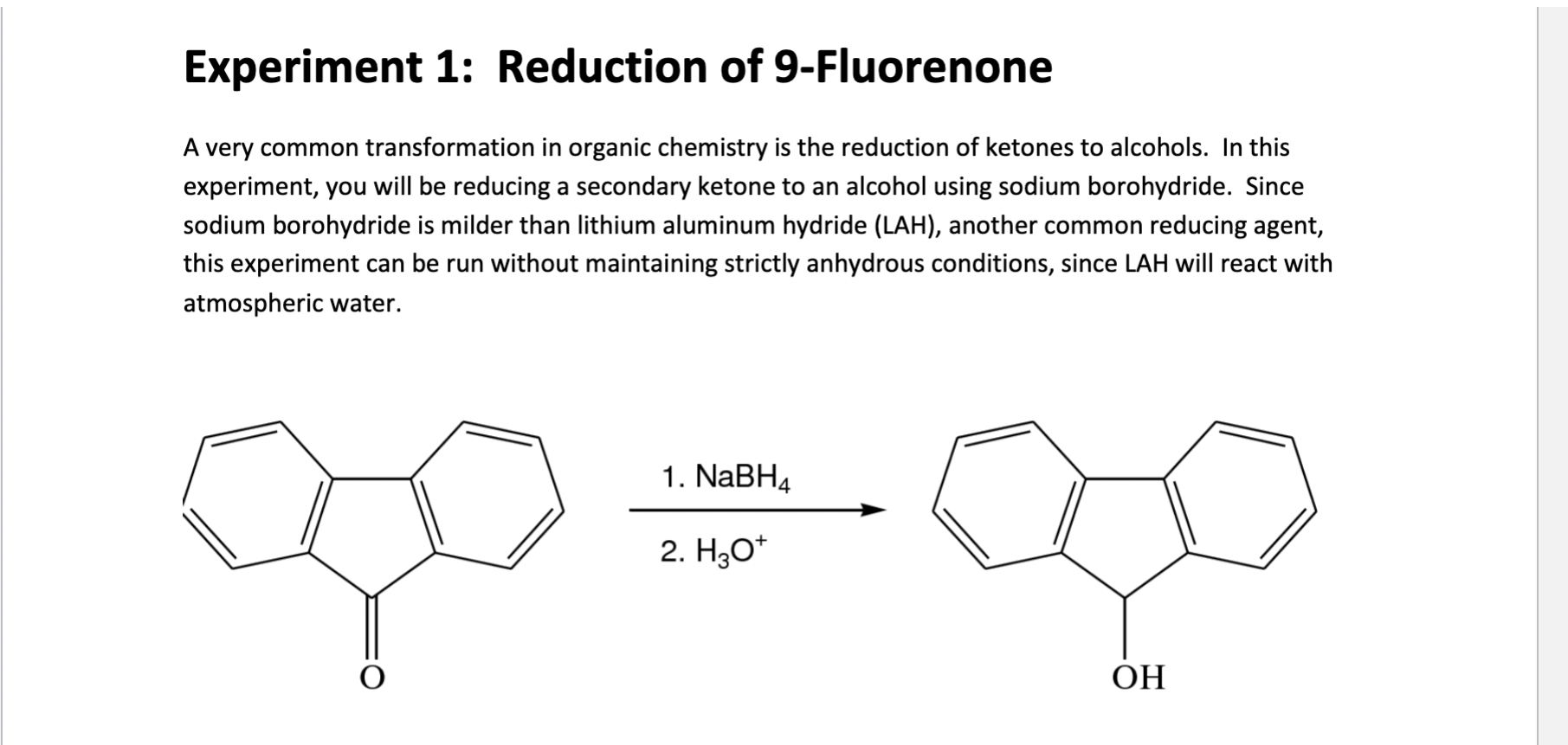

Experiment 1: Reduction of 9-Fluorenone A very common transformation in organic chemistry is the reduction of ketones to alcohols. In this experiment, you will be reducing a secondary ketone to an alcohol using sodium borohydride. Since sodium borohydride is milder than lithium aluminum hydride (LAH), another common reducing agent, this experiment can be run without maintaining strictly anhydrous conditions, since LAH will react with atmospheric water. 1. NaBH4 2. H2O+ OH Post-Lab Analysis 1. What is the mechanism for this reaction? 2. What are the advantages and disadvantages of using sodium borohydride compared to LAH? 3. Will the product of this reaction differ if LAH is used instead of sodium borohydride
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


