Question: please i need solution in one hour Example Use the RK equation to estimate the specific volume of a mixture containing la 26.92 wt% propane
please i need solution in one hour
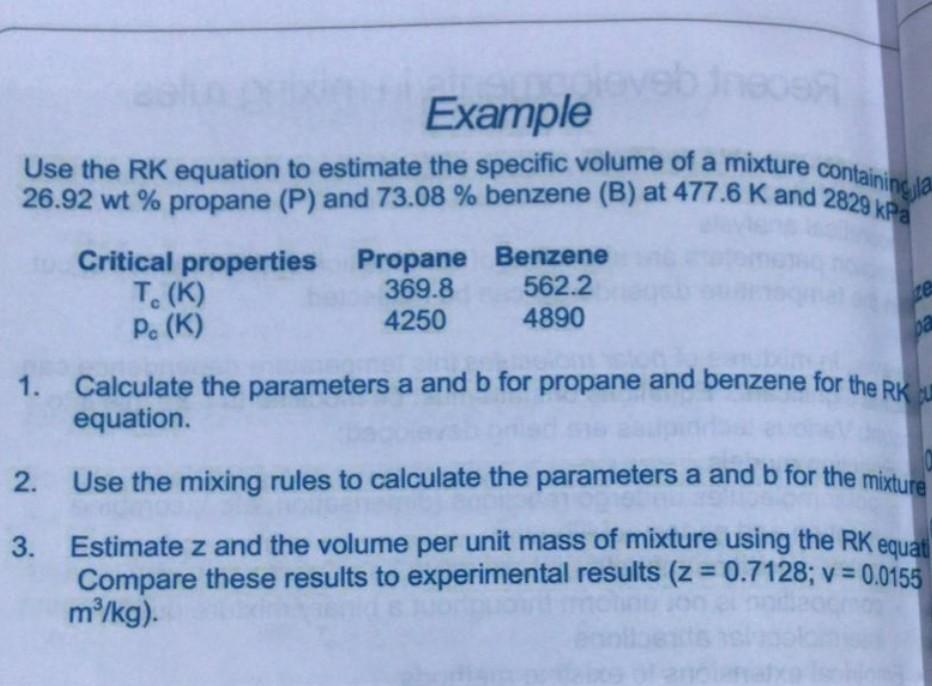
Example Use the RK equation to estimate the specific volume of a mixture containing la 26.92 wt% propane (P) and 73.08 % benzene (B) at 477.6 K and 2829 kPa Critical properties Propane Benzene T. (K) 369.8 562.2 P. (K) 4250 4890 1. Calculate the parameters a and b for propane and benzene for the RK. equation. 2. Use the mixing rules to calculate the parameters a and b for the mixture 3. Estimate z and the volume per unit mass of mixture using the RK equat Compare these results to experimental results (z = 0.7128; v = 0.0155 m/kg)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


