Question: please i need your help Use the van der Waals equation to calculate the pressure exerted by 1.200mol of Cl2 in a volume of 4.870L
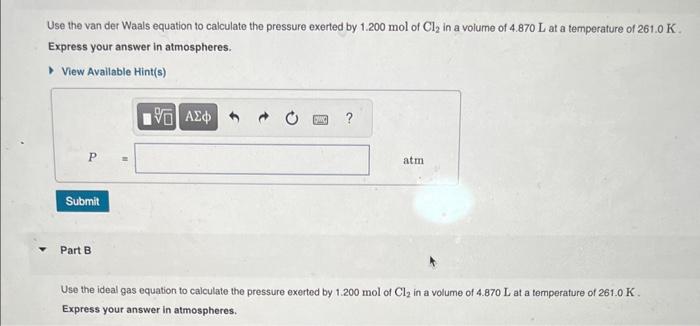
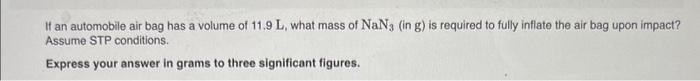
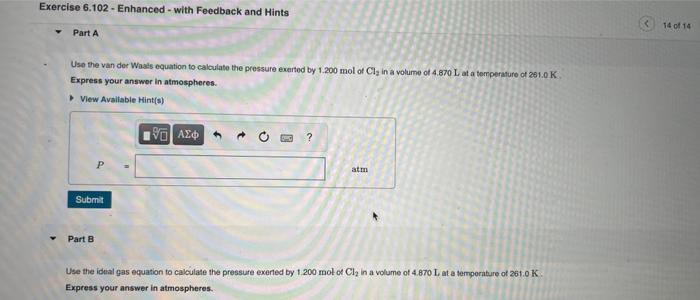
Use the van der Waals equation to calculate the pressure exerted by 1.200mol of Cl2 in a volume of 4.870L at a temperature of 261.0K. Express your answer in atmospheres. Part B Use the ideal gas equation to calculate the pressure exerted by 1.200mol of Cl2 in a volume of 4.870L at a temperature of 261.0K. Express your answer in atmospheres. If an automobile air bag has a volume of 11.9L, what mass of NaN3 (in g ) is required to fully inflate the air bag upon impact? Assume STP conditions. Express your answer in grams to three significant figures. Use the van der Waaks equation to calculate the pressure exarted by 1.200mol of Cl2 in a volume of 4.870L at a temperature of 251.0K. Express your answor in atmospheres. Part 8 Use the ideal gas equation to calculate the pressure exeried by 1.200mot of Cl2 in a volume of 4.870L at a temporiture of 261.0 K. Express your answer in atmospheres
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


