Question: Please identify every number what is given and the unknown, also please open the link below. and follow how it solve, use the same variable
Please identify every number what is given and the unknown, also please open the link below. and follow how it solve, use the same variable in the module and the steps it used. example.


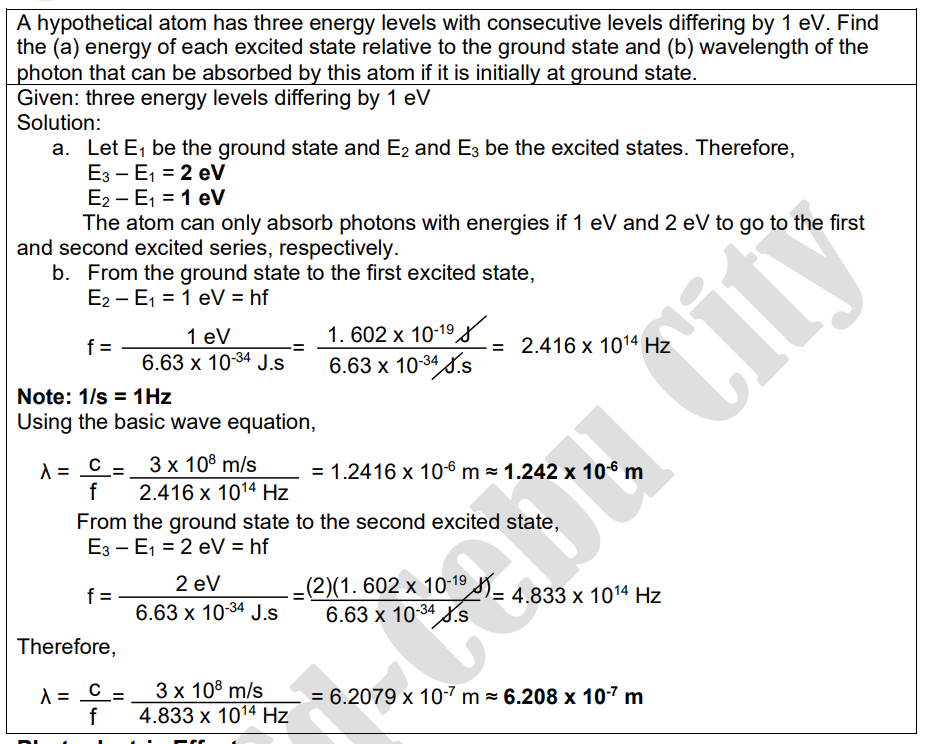
C. Instructions: Solve the following problems by identifying the given and unknown. Then, show your solution/s for each problem. Enclose in a box the final answer. Use a separate sheet of paper. 1. What is the radius of the nucleus of 24 Si? What is its volume? 2. A hypothetical atom has three energy levels with consecutive levels differing by 1.2 eV. Find the (a) energy of each excited state relative to the ground state and (b) wavelength of the photon that can be absorbed by this atom if it is initially at ground state. 3. The work function of a metal is 2.6 eV. What is the longest wavelength of radiation that can eject a photoelectron from this metal? 4. A radioactive sample has a decay constant of 0.2193/min. (a) What is its half-life? (b) If at the start of an experiment, there are 3.2 x 1015 nuclei present, how much will remain after 1.0 h?Lesson 2 What's More Ill. Problem-solving 1. R = 3.46x 10-15 m V = 1.73 x 1043 m3 2. E3 - E1 = 2.4 eV E2 - E1 = 1.2 eV Ground state to first excited state: A = 1.03 x 106 m Ground state to second excited state: A = 5.17 x 107 m 3. A = 4.77 x 107 m or 477 nm 4. T 12 = 3.16 mins N = 6.18x 109 nucleiA hypothetical atom has three energy levels with consecutive levels differing by 1 ev. Find the (a) energy of each excited state relative to the ground state and (b) wavelength of the photon that can be absorbed by this atom if it is initially at ground state. Given: three energy levels differing by 1 ev Solution: a. Let E, be the ground state and E2 and Es be the excited states. Therefore, E3 - E1 = 2 eV E2 - E1 = 1 eV The atom can only absorb photons with energies if 1 ev and 2 ev to go to the first and second excited series, respectively. b. From the ground state to the first excited state, E2 - E1 = 1 eV = hf f = 1 ev 1. 602 x 10-19 J _ 2.416 x 1014 Hz 6.63 x 10-34 J.S 6.63 x 10-34 J.S Note: 1/s = 1Hz Using the basic wave equation, A= C= 3x 108 m/s = 1.2416 x 105 m = 1.242 x 106 m f 2.416 x 1014 Hz From the ground state to the second excited state, E3 - E1 = 2 eV = hf 2 ev f = (2)(1. 602 x 10-19 J)= 4.833 x 1014 Hz 6.63 x 10-34 J.S 6.63 x 10-34 J.s Therefore, A= C = 3 x 108 m/s = 6.2079 x 107 m = 6.208 x 107 m f 4.833 x 1014 Hz
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


