Question: Please if you are using graph for solution, add them to your solution so it can be understandable. H2+C3H8CH4+C2H6 Schultz and Linden have studied the
 Please if you are using graph for solution, add them to your solution so it can be understandable.
Please if you are using graph for solution, add them to your solution so it can be understandable.
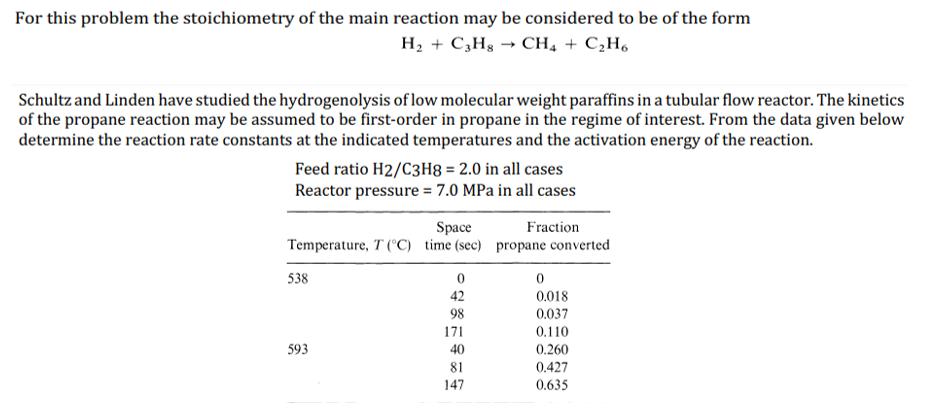
H2+C3H8CH4+C2H6 Schultz and Linden have studied the hydrogenolysis of low molecular weight paraffins in a tubular flow reactor. The kinetics of the propane reaction may be assumed to be first-order in propane in the regime of interest. From the data given below determine the reaction rate constants at the indicated temperatures and the activation energy of the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


