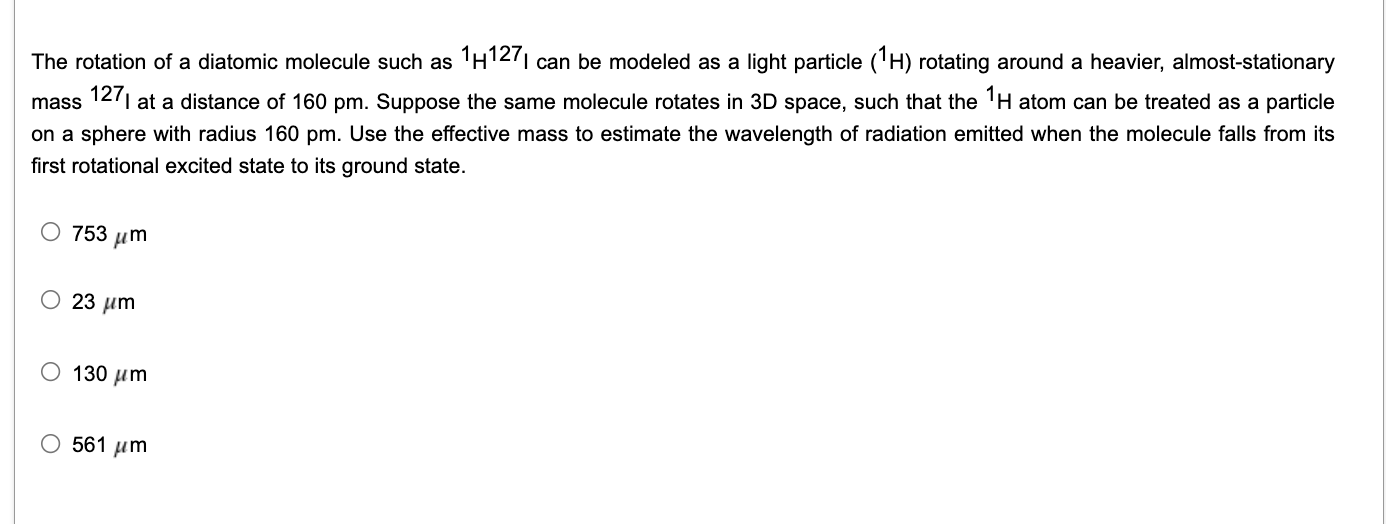
Question: Please include complete solution The rotation of a diatomic molecule such as 1H1271 can be modeled as a light particle (1H) rotating around a heavier,
 Please include complete solution
Please include complete solution
The rotation of a diatomic molecule such as 1H1271 can be modeled as a light particle (1H) rotating around a heavier, almost-stationary mass 1271 at a distance of 160 pm. Suppose the same molecule rotates in 3D space, such that the 1H atom can be treated as a particle on a sphere with radius 160 pm. Use the effective mass to estimate the wavelength of radiation emitted when the molecule falls from its first rotational excited state to its ground state. O 753 um O 23 um 130 m O 561 um
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


