Question: Please include notes beside relevant steps in solution so I can understand why certain things are done. Thank you. 3. The aqueous phase acid catalysed
Please include notes beside relevant steps in solution so I can understand why certain things are done. Thank you.
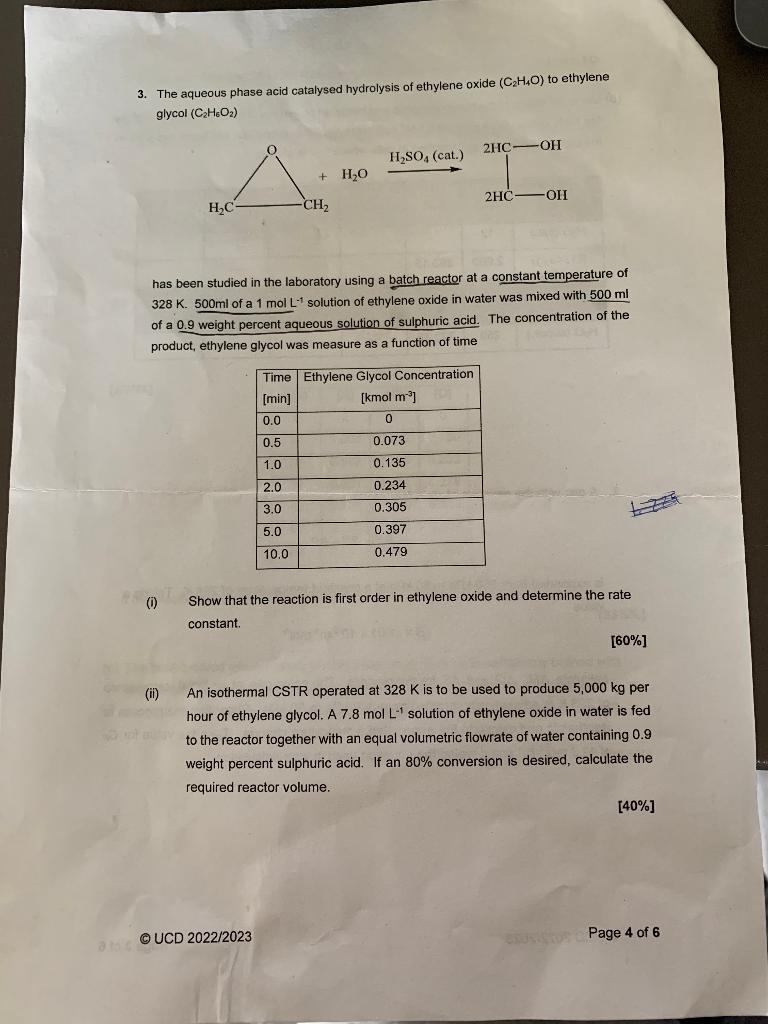
3. The aqueous phase acid catalysed hydrolysis of ethylene oxide (C2H4O) to ethylene glycol (C2H6O2) has been studied in the laboratory using a batch reactor at a constant temperature of 328K. 500ml of a 1molL1 solution of ethylene oxide in water was mixed with 500mI of a 0.9 weight percent aqueous solution of sulphuric acid. The concentration of the product, ethylene glycol was measure as a function of time (i) Show that the reaction is first order in ethylene oxide and determine the rate constant. [60%] (ii) An isothermal CSTR operated at 328K is to be used to produce 5,000kg per to the reactor together with an equal volumetric flowrate of water containing 0.9 weight percent sulphuric acid. If an 80% conversion is desired, calculate the required reactor volume. [40\%] 3. The aqueous phase acid catalysed hydrolysis of ethylene oxide (C2H4O) to ethylene glycol (C2H6O2) has been studied in the laboratory using a batch reactor at a constant temperature of 328K. 500ml of a 1molL1 solution of ethylene oxide in water was mixed with 500mI of a 0.9 weight percent aqueous solution of sulphuric acid. The concentration of the product, ethylene glycol was measure as a function of time (i) Show that the reaction is first order in ethylene oxide and determine the rate constant. [60%] (ii) An isothermal CSTR operated at 328K is to be used to produce 5,000kg per to the reactor together with an equal volumetric flowrate of water containing 0.9 weight percent sulphuric acid. If an 80% conversion is desired, calculate the required reactor volume. [40\%]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


