Question: Please include the programming codes if possible Question 1: The dissolution of copper sulfide in aqueous nitric acid is described by the following chemical equation:
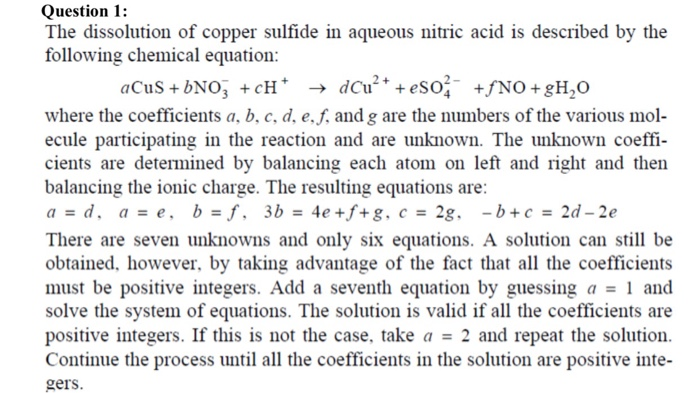
Question 1: The dissolution of copper sulfide in aqueous nitric acid is described by the following chemical equation: aCuS+bNO3 +cH+ + dCu+ + eSO2- +fNO +gH,O where the coefficients a, b, c, d, e, f, and g are the numbers of the various mol- ecule participating in the reaction and are unknown. The unknown coeffi- cients are determined by balancing each atom on left and right and then balancing the ionic charge. The resulting equations are: a = d, a = e, b = f, 3b = 4e +f+g, c = 2g, - b + c = 2d - 2e There are seven unknowns and only six equations. A solution can still be obtained, however, by taking advantage of the fact that all the coefficients must be positive integers. Add a seventh equation by guessing a = 1 and solve the system of equations. The solution is valid if all the coefficients are positive integers. If this is not the case, take a = 2 and repeat the solution. Continue the process until all the coefficients in the solution are positive inte- gers
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


