Question: please indicate tge answer Use the information below to determine whether or not a reaction miature in which the partial pressures of PCl,Cl2, and PCl
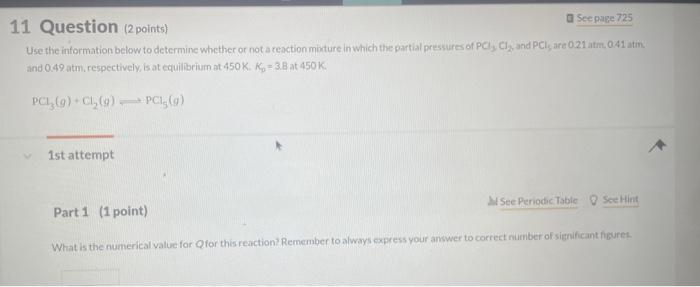
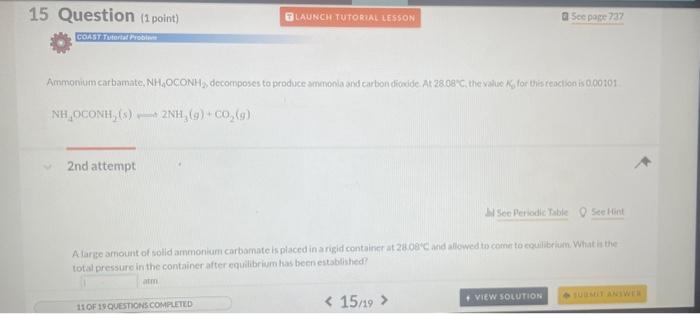
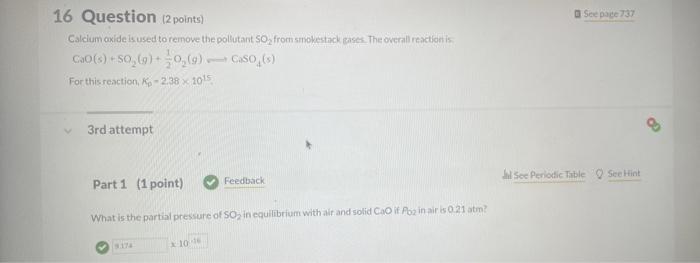
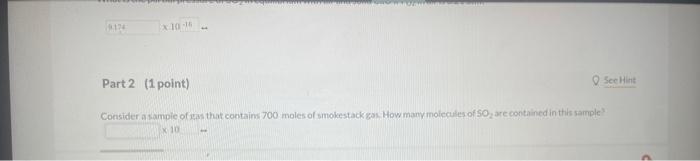
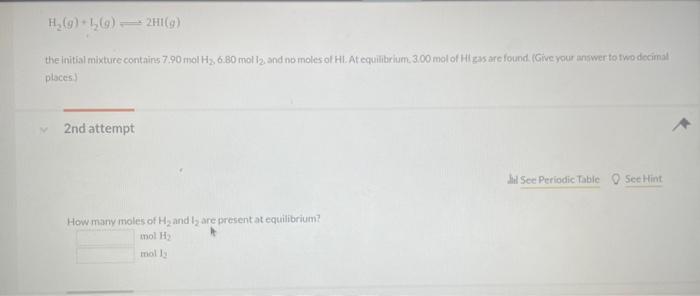
Use the information below to determine whether or not a reaction miature in which the partial pressures of PCl,Cl2, and PCl are 0.21 atim, 041 atin. and 0.49 atm. respectively, is at equilibriurn at 450K,Kp=3.8 at 450K. PCl3(g)+Cl2(g)PCl9(g) 1 st attempt Part 1 (1 point) What is the numerical value for Qfor this reaction' Remember to always express your answer to correct number of significant figures. NH4OCONH2(s)2NH3(g)+CO2(g) 2nd attempt. A large amouit of solld ammonium carbamate is placed in a rigid container at 26 . Oave and allowed to comere to ceuilibriain. What in the total oressure in the container after equilibrium has been establiahed? CaO(s)+SO2(g)+21O2(g)CaSO4(s) For this reaction, kp2.381015. 3rdattempt Part 1 (1 point) What is the partial pressure of SO2 in equilibrium with alr and solid CaO if f2 in air is 021atm ? Consider asainpie of agas that contains 700 moles of smokestack gas. How many molecules of 502 are contained in thits sample' H2(g)+I2(g)2HI(g) places.) 2nd attempt How many moles of H2 and l2 are present at equilibrium? SecHint molH2 mal ly
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


