Question: Please let me know which one to slide it too. There are three sets of sketches below, showing the same pure molecular compound (sulfur dioxide,
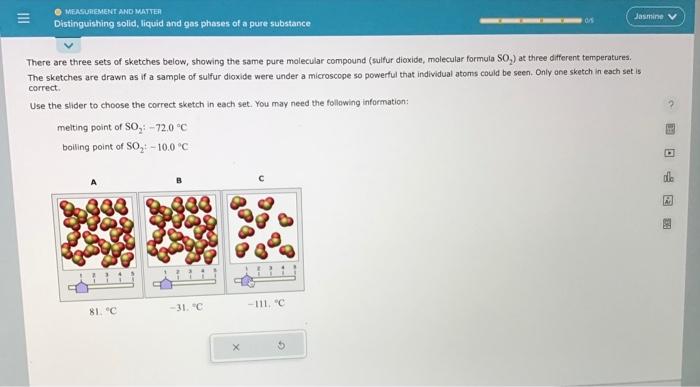
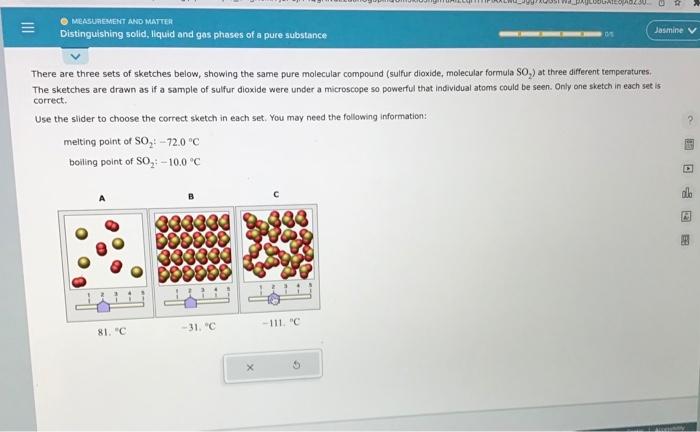
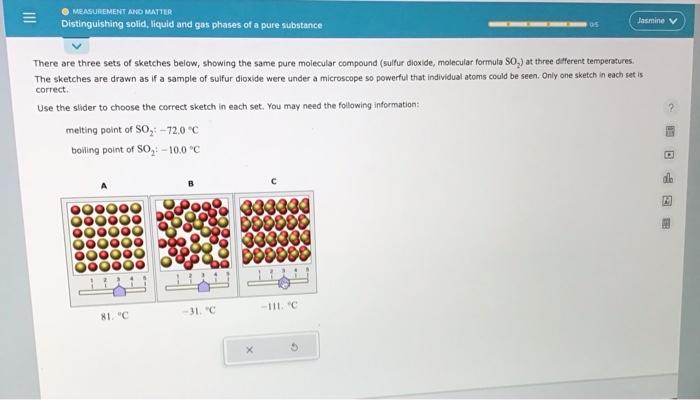
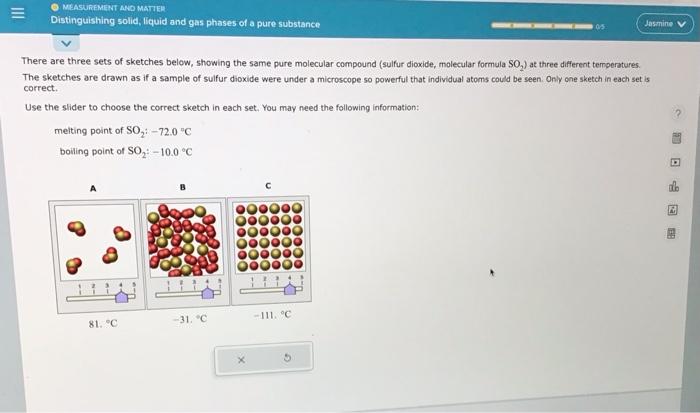
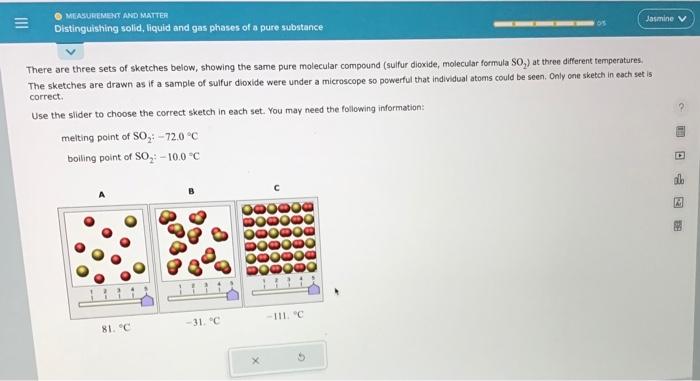
There are three sets of sketches below, showing the same pure molecular compound (sulfur dioxide, molecular formula SO2 ) at three different temperatures. The sketches are drawn as if a sample of sulfur dioxide were under a microscope so powerful that individual atoms could be seen. Only ane sketch in each set is carrect. Use the slider to choose the correct sketch in each set. You may need the follawing information: melting point of SO2:72.0C boiling point of SO2+100C There are three sets of sketches below, showing the same pure molecular compound (sulfur dioxide, molecular formula SO S2 ) at three different temperatures. The sketches are drawn as if a sample of sulfur dioxide were under a microscope so powerful that individual atoms could be seen. Only ane sketch in each set is correct. Use the slider to choose the correct sketch in each set, You may need the following information: melting point of SO2:72.0C boiling point of SO2;10.0C There are three sets of sketches below, showing the same pure molecular compound (sulfur dioxide, molecular formula SOO2 ) at three different temperetures. The sketches are drawn as if a sample of sulfur dioxide were under a microscope so powerful that individual atoms could be seen. Only one sketch in each set is correct. Use the slider to choose the correct sketch in each set. You may need the following information: meiting point of SO272.0C boiling point of SO2:10.0C There are three sets of sketches below, showing the same pure molecular compound (sulfur dioxide, molecular formula SO, The sketches are drawn as if a sample of sulfur dioxide were under a microscope so powerful that individual atoms could be seen. Only one sketch in each set is correct. Use the slider to choose the correct sketch in each set. You may need the following information: melting point of SO2:72.0nC boiling point of SO2:10.0C There are three sets of sketches below, showing the same pure molecular compound (suifur dioxide, molecular formula SO2 ) at three different temperatures. The sketches are drawn as if a sample of sulfur dioxide were under a microscope so powerful that individual atoms could be seen, Only one sketch in each set is correct. Use the slider to choose the correct sketch in each set. You may need the following information: melting point of SO272.0C boiling point of SO2=10.0=C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


