Question: please make a mathematical model, transfer function and control the process. This is from the book of Bulletin of Chemical Society Japan Fig. 3.Schematic illustration
please make a mathematical model, transfer function and control the process. This is from the book of Bulletin of Chemical Society Japan
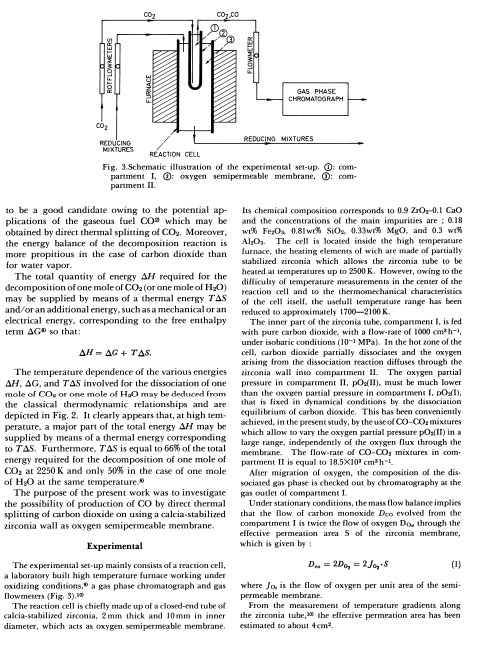
Fig. 3.Schematic illustration of the experimental set-up. (1): com- parment I, (9): oxygen semipermeable membrane, (): comparment II. to be a good candidate owing to the potential applications of the gaseous fuel CO2 which may be obtained by direct thermal splitting of CO2. Moreover, the energy balance of the decomposition reaction is more propitious in the case of carbon dioxide than for water vapor. The total quantity of energy H required for the decomposition of one mole of CO2 (or one mole of H2O ) may be supplied by means of a thermal energy TS and/or an additional energy, such as a mechanical or an electrical energy, corresponding to the free enthalpy term G0 so that: H=G+TS The temperature dependence of the various encrgies H,G, and TS involved for the dissociation of one mole of CO0 or one mole of H0O may be deduced from the classical thermodynamic relationships and are depicted in Fig. 2. It clearly appears that, at high temperature, a major part of the total energy H may be supplied by means of a thermal energy corresponding to TS. Furthermore, TS is equal to 66% of the total energy required for the decomposition of one mole of CO2 at 2250K and only 50% in the case of one mole of H2O at the same temperature. The purpose of the present work was to investigate the possibility of production of CO by direct thermal splitting of carbon dioxide on using a calcia-stabilized zirconia wall as oxygen semipermeable membrane- Experimental The experimental set-up mainly consists of a reaction cell, a laboratory built high temperature furnace working under oxidizing conditions," a gas phase chromatograph and gas flowmeters (Fig, 3), 100 The reaction cell is chiefly made up of a closed-end tube of calcia-sabilized zirconia, 2mm thick and 10mm in inner diameter, which acts as oxygen semipermeable membrane. Its chemical composition corresponds to 0.9ZrO20.1CaO and the concentrations of the main impurities are ; 0.18 wt\% Fe2O20.81 wt 02SiO2,0.33 wi MgO4, and 0.3 wth Al2O3. The cell is located inside the high temperature furnace, the beating elements of wich are made of partially stabilized zirconia which allows the zirconia tube to be heaced at temperatures up to 2500K. However, owing to the difticulny of temperature measurements in the center of the reaction cell and to the thermomechanical characteristics of the cell isself, the usefull temperature range has been reduced to approximately 17002100K. The inner part of the zirconia tube, compartment I, is fed with pure carbon dioxide, with a flow-rate of 1000cm2h1, under isobaric conditions ( 101MPa ). In the hot zone of the cell, carbon dioxide partially dissociates and the oxygen arising from the dissociation reaction diffuses through the circonia wall into compartment II. The oxygen partial pressure in compartment II, pO (II), must be much lower than the oxveet partial pressure in comparument I,pO(1), that is fixed in dyramical conditions by the dissociation equilibrium of carbon dioxide. This bas been conveniently achieved, in the present study, by the use of OOCO2 mixtures which allow to vary the oxygen partial pressure pOOs(II) in a large range, independently of the oxygen flux through the membrane. The flow-rate of COCO2 mixtures in compartment II is equal to 18.5103cm2h1. Niter migration of oxygen, the composition of the dis. sociated gas phase is checked out by chromatography at the gas outlet of compartment I. Under stationary conditions, the mass flow balance implies that the flow of arbon monoxide Dcoevolvedfromthe compartment I is twice the flow of exygen DD, through the effective permeation area S of the zirconia membrane, which is given by : Des=2D02=2J02+S where J0 is the flow of axygen per unit area of the semipermeable membrane. From the measurement of temperature gradients along the zirconia tube, 20 the effective permeation area has been estimated to about 4cm2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


