Question: please make simple 29. Consider the reactions and their respective equilibrium constants: NO(g)+21Br2(g)NOBr(g)+2NO(g)N2(g)+O2(g)KK=5.3=2.11030 Use these reactions and their equilibrium constants to predict the equilibrium constant
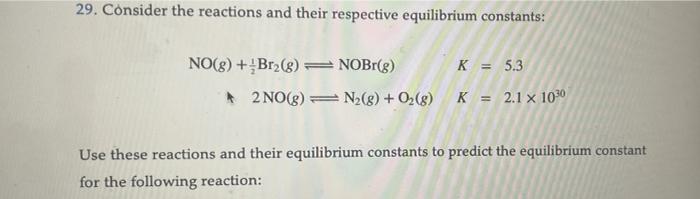
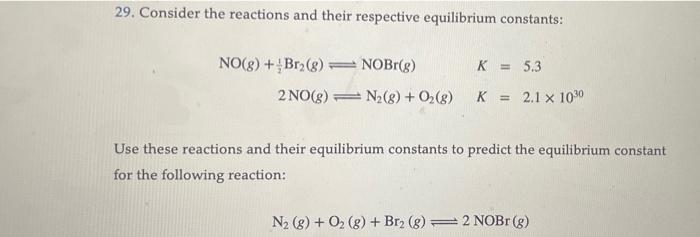
29. Consider the reactions and their respective equilibrium constants: NO(g)+21Br2(g)NOBr(g)+2NO(g)N2(g)+O2(g)KK=5.3=2.11030 Use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: 29. Consider the reactions and their respective equilibrium constants: NO(g)+21Br2(g)NOBr(g)2NO(g)N2(g)+O2(g)KK=5.3=2.11030 Use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: N2(g)+O2(g)+Br2(g)2NOBr(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


