Question: **Please make the solution clear and easy to understand** Thank you Problem 3 Liquid Acetone (C3H60) is fed at a rate of 400 L/min into
**Please make the solution clear and easy to understand** Thank you
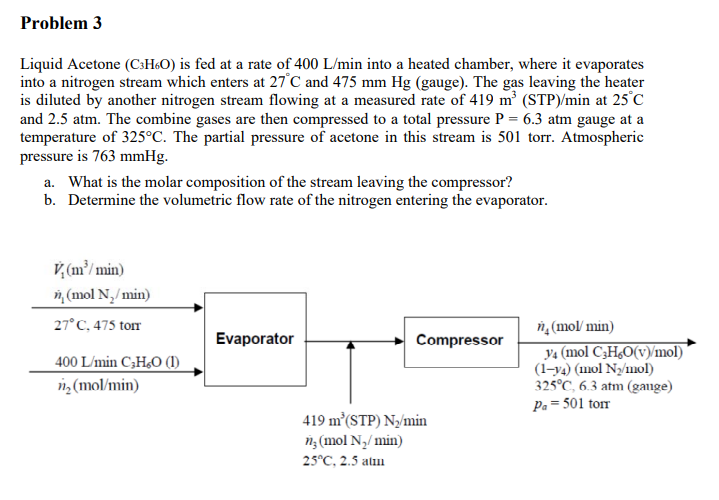
Problem 3 Liquid Acetone (C3H60) is fed at a rate of 400 L/min into a heated chamber, where it evaporates into a nitrogen stream which enters at 27C and 475 mm Hg (gauge). The gas leaving the heater is diluted by another nitrogen stream flowing at a measured rate of 419 m (STP)/min at 25C and 2.5 atm. The combine gases are then compressed to a total pressure P = 6.3 atm gauge at a temperature of 325C. The partial pressure of acetone in this stream is 501 torr. Atmospheric pressure is 763 mmHg. a. What is the molar composition of the stream leaving the compressor? b. Determine the volumetric flow rate of the nitrogen entering the evaporator. V(m/min) n (mol N,/min) 27C. 475 torr Evaporator Compressor 400 L/min C3H20 (1) 1,(mol/min) n (mol/min) ya (mol C3H6O(v)/mol) (1-ya) (mol Nz/mol) 325C, 6.3 atm (gange) Pa = 501 torr 419 m (STP) Ny/min nz (mol N/min) 25C, 2.5 alin
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


