Question: Please note the final one is SN and not Zn, and please show work For each of the metals listed in the table, compute the
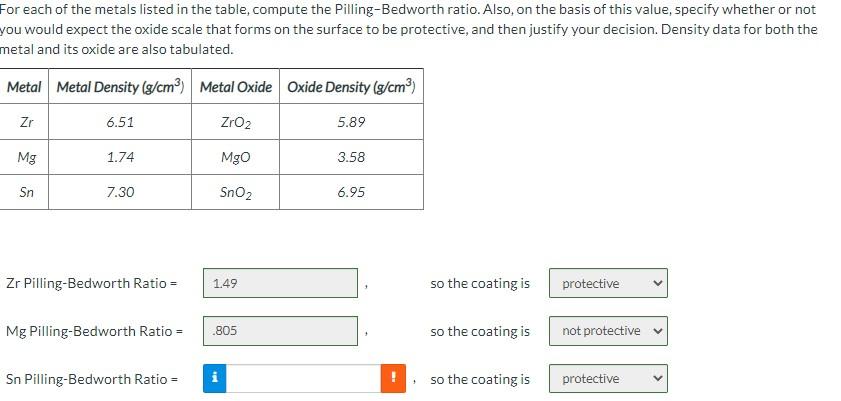
Please note the final one is SN and not Zn, and please show work
For each of the metals listed in the table, compute the Pilling-Bedworth ratio. Also, on the basis of this value, specify whether or not you would expect the oxide scale that forms on the surface to be protective, and then justify your decision. Density data for both the metal and its oxide are also tabulated. Metal Metal Density (g/cm) Metal Oxide Oxide Density (g/cm) Zr 6.51 ZrO2 5.89 Mg 1.74 Mgo 3.58 Sn 7.30 SnO2 6.95 Zr Pilling-Bedworth Ratio = 1.49 so the coating is protective Mg Pilling-Bedworth Ratio = .805 so the coating is not protective Sn Pilling-Bedworth Ratio = so the coating is protective
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


