Question: please only answer if gonna answer all A compound is 53.31% C, 11.18% H, and 35.51% O by mass. What is its empirical formula? Insert
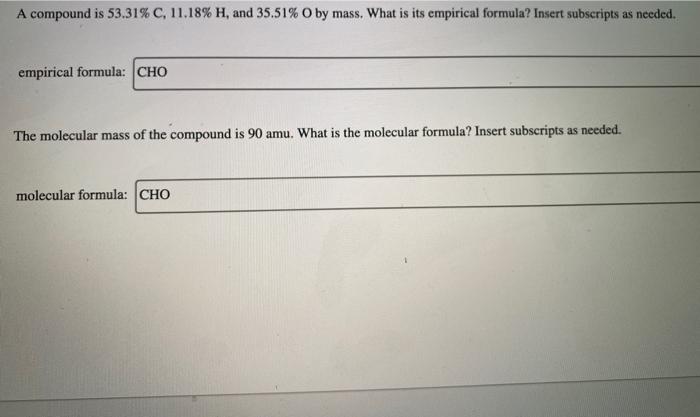
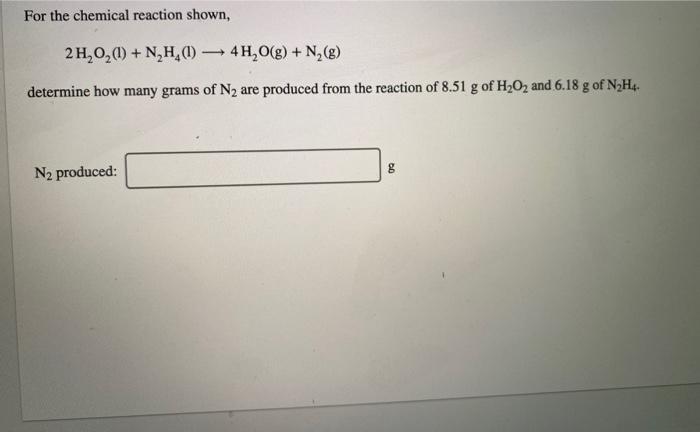
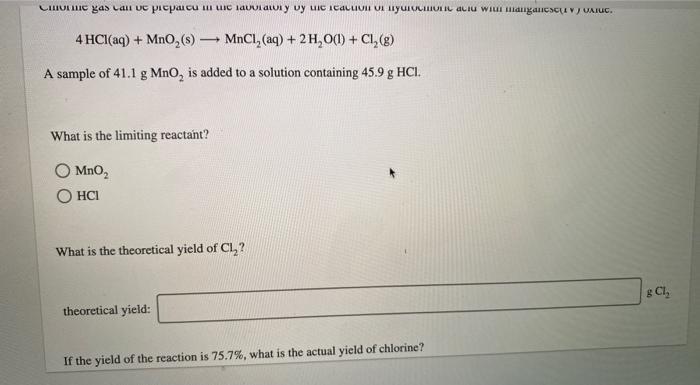
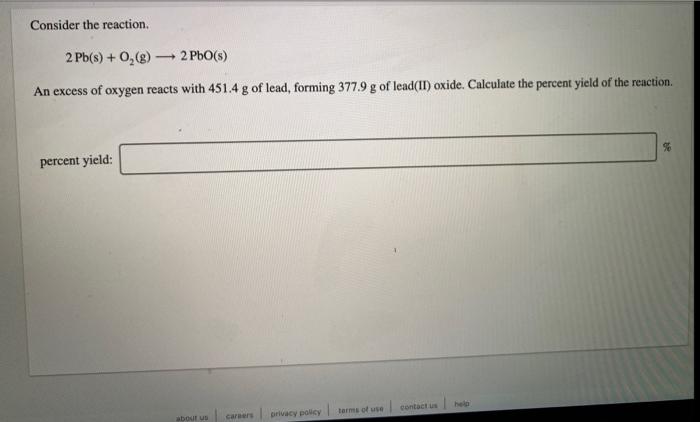

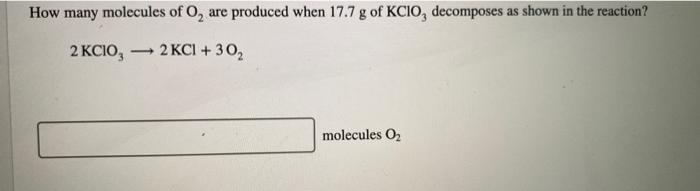
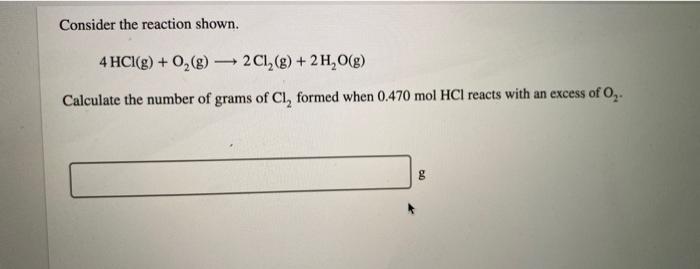
A compound is 53.31% C, 11.18% H, and 35.51% O by mass. What is its empirical formula? Insert subscripts as needed. empirical formula: CHO The molecular mass of the compound is 90 amu. What is the molecular formula? Insert subscripts as needed. molecular formula: CHO For the chemical reaction shown, 2H,02(1) + N,H,(1) 4H2O(g) + N, (g) determine how many grams of N2 are produced from the reaction of 8.51 g of H2O2 and 6.18 g of N2H4. N2 produced: 8 LHC yas cauc piepai CU LI Lic lau LUI y uy Lic ICALLIVIUL BULUCTUULIL ALIU WILL Hauganesc Y VAIUC. 4 HCl(aq) + MnO,() MnCl(aq) + 2H2O(l) + Cl (8) A sample of 41.1 g Mno, is added to a solution containing 45.9 g HCI. What is the limiting reactant? Mno, HCI What is the theoretical yield of CI? BCI, theoretical yield: If the yield of the reaction is 75.7%, what is the actual yield of chlorine? Consider the reaction. 2 Pb(s) + 0,(8) 2PbO() An excess of oxygen reacts with 451.4 g of lead, forming 377.9 g of lead(II) oxide. Calculate the percent yield of the reaction. 98 percent yield: privacy policy terms of use contact us about care Consider the combustion reaction for octane (C,H,g), which is a primary component of gasoline. 2CH. + 2502 16CO, +18 H,0 How many moles of Co, are emitted into the atmosphere when 15.6g C,H, is burned? CO, emitted: mol CO, How many molecules of O, are produced when 17.7 g of KCIO, decomposes as shown in the reaction? 2 KCIO, - 2 KCI + 302 molecules O2 Consider the reaction shown. 4 HCl(g) + O2(g) 2C12(g) + 2 H2O(g) Calculate the number of grams of Cl, formed when 0.470 mol HCl reacts with an excess of O, 8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


