Question: Please please answer wll 4! I dont get it Consider the following system at equilibrium where H=111kJ, and Kc=6.30, at 723K: 2NH3(g)N2(g)+3H2(g) If the VOLUME
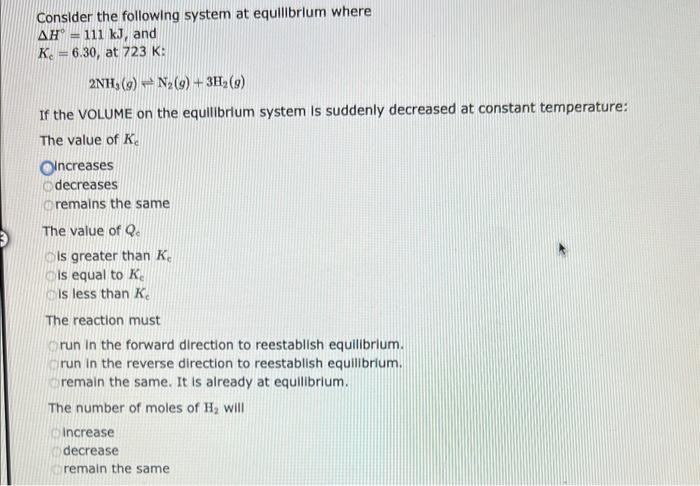
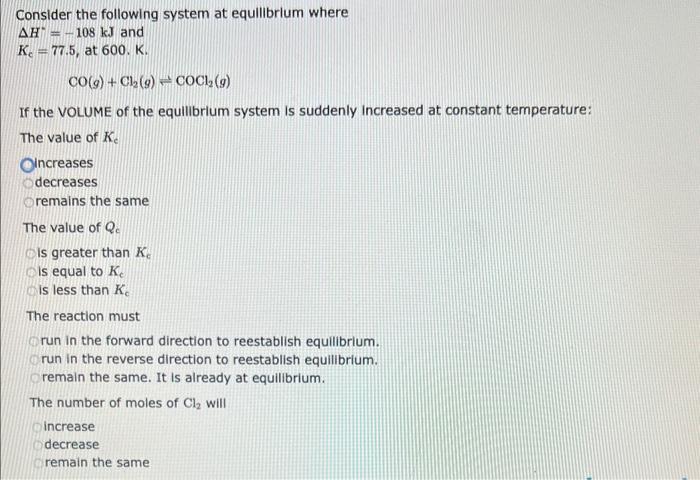


Consider the following system at equilibrium where H=111kJ, and Kc=6.30, at 723K: 2NH3(g)N2(g)+3H2(g) If the VOLUME on the equilibrium system is suddenly decre The value of Kc ncreases decreases remains the same The value of Qe is greater than Kc is equal to Ke is less than Kc The reaction must run in the forward direction to reestablish equilibrium. run in the reverse direction to reestablish equilibrium. remain the same. It is already at equilibrium. The number of moles of H2 will increase Consider the following system at equllibrium where H=108kJandKc=77.5,at600.K.CO(g)+Cl2(g)COCl2(g) If the VOLUME of the equilibrium system is suddenly incre The value of Kc ncreases decreases remains the same The value of Qc is greater than Kc is equal to Kc is less than Kc The reaction must run in the forward direction to reestablish equilibrium. run in the reverse direction to reestablish equilibrium. remain the same. It is already at equilibrium. The number of moles of Cl2 will increase Consider the following system at equillbrium where H=18.8kJ, and Kc=1.05101, at 350K: 2CH2Cl2(g)CH4(g)+CCl4(g) If the temperature on the equilibrium system is suddenly decreased: The value of Kc ncreases decreases remains the same The value of Qe is less than Kc Is greater than Kc is equal to Kc The reaction must run in the forward direction to reestablish equilibrium run in the reverse direction to reestablish equilibrium remain in the current position, since it is already at equilibrium The concentration of CCl4 will increase decrease remain the same Consider the following system at equilibrium where H=198kJ, and Kc=2.90102, at 1150K: 2SO3(g)2SO2(g)+O2(g) If the temperature on the equilibrium system is suddenly decreased: The value of Kc ncreases decreases remains the same The value of Qc is less than Ke is greater than Kc is equal to Kc The reaction must run in the forward direction to reestablish equilibrium run in the reverse direction to reestablish equilibrium remain in the current position, since it is already at equilibrium The concentration of O2 will increase decrease remain the same
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


