Question: Please post all steps 6. Find the appropriate equilibrium constant (Kp) for the reaction below under the following conditions. 21SnO2(s)+H2(g)H2O(g)+21Sn(s) a. At 650C the equilibrium
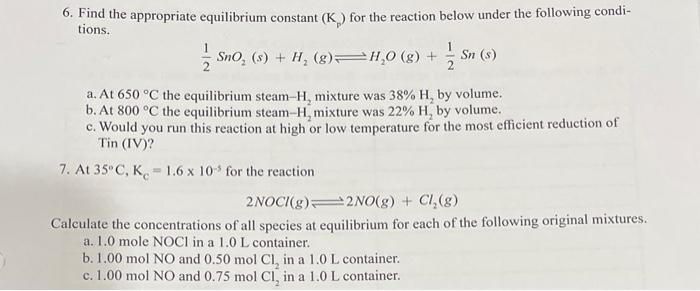
6. Find the appropriate equilibrium constant (Kp) for the reaction below under the following conditions. 21SnO2(s)+H2(g)H2O(g)+21Sn(s) a. At 650C the equilibrium steam- H2 mixture was 38%H2 by volume. b. At 800C the equilibrium steam- H2 mixture was 22%H2 by volume. c. Would you run this reaction at high or low temperature for the most efficient reduction of Tin (IV)? 7. At 35C,KC=1.6105 for the reaction 2NOCl(g)2NO(g)+Cl2(g) Calculate the concentrations of all species at equilibrium for each of the following original mixtures. a. 1.0 mole NOCl in a 1.0L container. b. 1.00molNO and 0.50molCl2 in a 1.0L container. c. 1.00molNO and 0.75molCl22 in a 1.0L container
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


