Question: please post step by step solution An inexperienced engineer tells you that they have installed a very efficient compressor on their plant, which compresses a
please post step by step solution
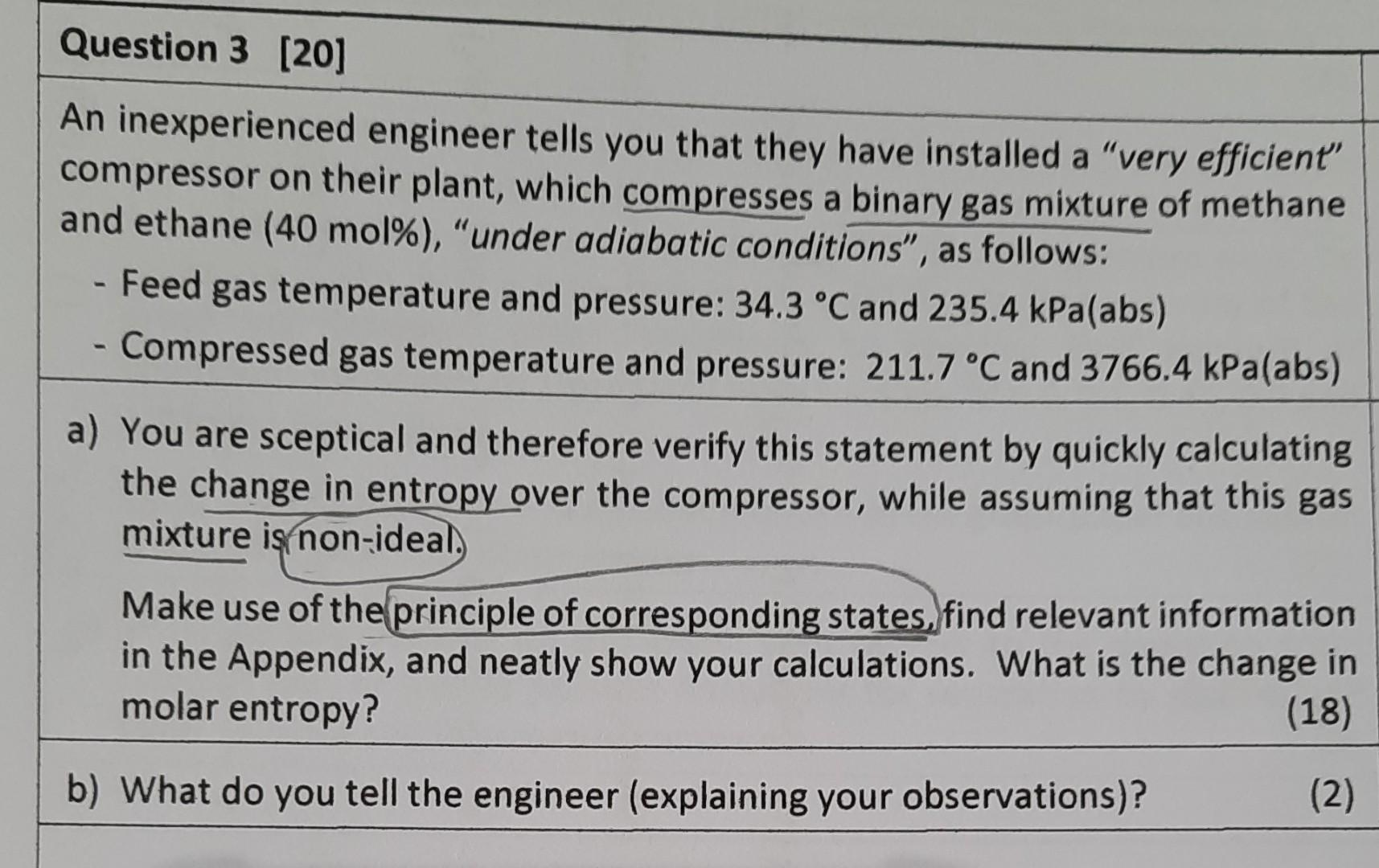
An inexperienced engineer tells you that they have installed a "very efficient" compressor on their plant, which compresses a binary gas mixture of methane and ethane (40 mol\%), "under adiabatic conditions", as follows: - Feed gas temperature and pressure: 34.3C and 235.4kPa(abs) - Compressed gas temperature and pressure: 211.7C and 3766.4kPa(abs) a) You are sceptical and therefore verify this statement by quickly calculating the change in entropy over the compressor, while assuming that this gas mixture is non-ideal. Make use of the principle of corresponding states, find relevant information in the Appendix, and neatly show your calculations. What is the change in molar entropy? (18) b) What do you tell the engineer (explaining your observations)? (2)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


