Question: Please post your own answer and not a copied answer from a previously answered post of this question. Exercise 3.9: Semipermeable membrane and chemical reaction
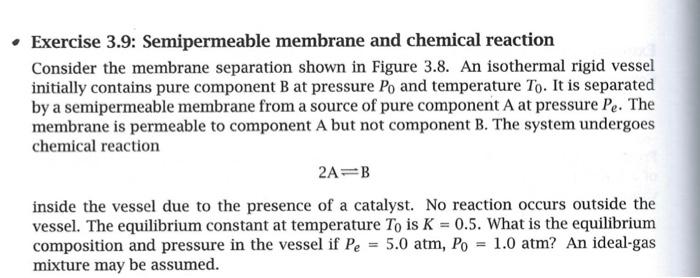
Exercise 3.9: Semipermeable membrane and chemical reaction Consider the membrane separation shown in Figure 3.8. An isothermal rigid vessel initially contains pure component B at pressure Po and temperature To. It is separated by a semipermeable membrane from a source of pure component A at pressure Pe. The membrane is permeable to component A but not component B. The system undergoes chemical reaction 2A=B inside the vessel due to the presence of a catalyst. No reaction occurs outside the vessel. The equilibrium constant at temperature To is K = 0.5. What is the equilibrium composition and pressure in the vessel if Pe = 5.0 atm, Po = 1.0 atm? An ideal-gas mixture may be assumed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


