Question: Please provide a solution an explanation to solve this probelm. Answer is False as seen. IT ? fine equilibrium mole fractions of Nz and O2
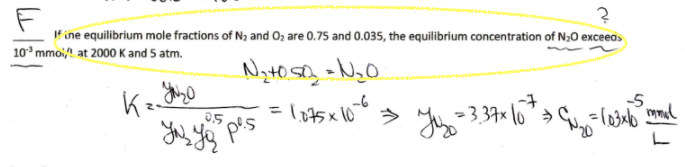
Please provide a solution an explanation to solve this probelm. Answer is False as seen.
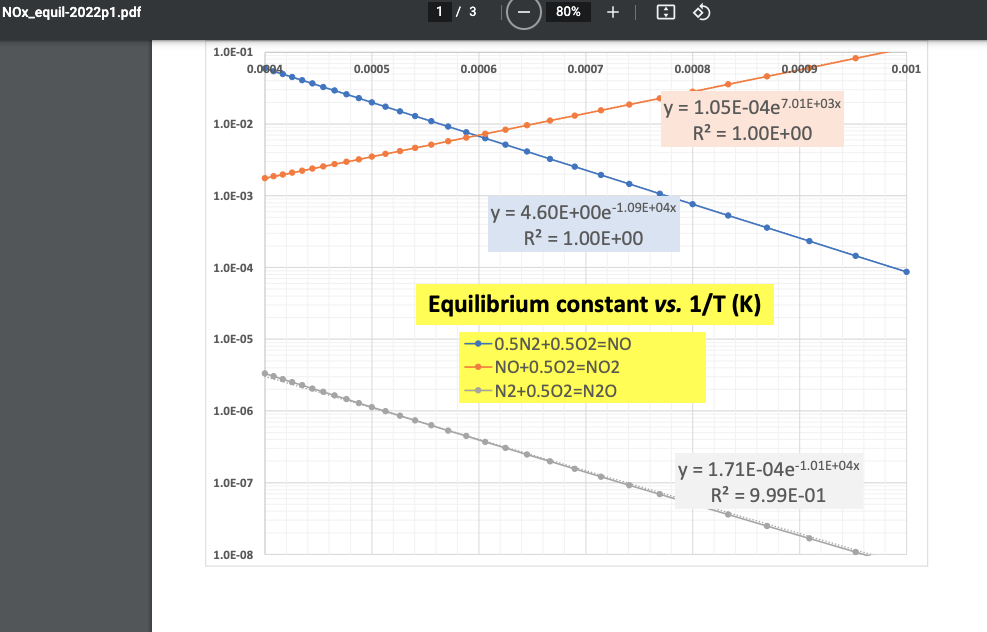
IT ? fine equilibrium mole fractions of Nz and O2 are 0.75 and 0.035, the equilibrium concentration of N20 exceeds 10 mmol at 2000 K and 5 atm. Nato so No -6 -5 0.5 - Kz Yazo = 105X106 > Yuyu = 3.374 10 *> Chi lo183xb ramal pos L NOx_equil-2022p1.pdf 1 / 3 80% + 1.0E-01 0.0094 0.0005 0.0006 0.0007 0.0008 0.0009 0.001 1.0E-02 y = 1.05E-04e7.01E+03x R2 = 1.00E+00 1.0E-03 y = 4.60E+00e-1.09E+04x R2 = 1.00E+00 1.0E-04 Equilibrium constant vs. 1/T (K) 1.0E-05 +-0.5N2+0.502=NO -NO+0.502=NO2 ---N2+0.502=N20 1.0E-06 1.0E-07 y = 1.71E-04e-1.01E+04x R2 = 9.99E-01 1.0E-08
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


