Question: PLEASE PROVIDE CLEAR HANDRAWING , AND ONLY CORRECT ANSWERS WOULD BE UPVOTED (Note: this is a practice question) Compound A is a branched-chain alcohol that
PLEASE PROVIDE CLEAR HANDRAWING , AND ONLY CORRECT ANSWERS WOULD BE UPVOTED (Note: this is a practice question)
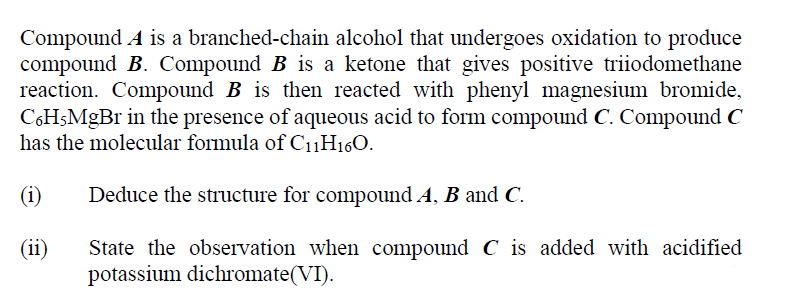
Compound A is a branched-chain alcohol that undergoes oxidation to produce compound B. Compound B is a ketone that gives positive triiodomethane reaction. Compound B is then reacted with phenyl magnesium bromide, C6H5MgBr in the presence of aqueous acid to form compound C. Compound C has the molecular formula of CuH160. (1) Deduce the structure for compound A, B and C. (ii) State the observation when compound C is added with acidified potassium dichromate(VI)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


