Question: PLEASE PROVIDE COMPREHENSIVE FULL ANSWER TO ALL PARTS. L D The second order reaction is taking place in 20 meters packed bed reactor of 1.5
PLEASE PROVIDE COMPREHENSIVE FULL ANSWER TO ALL PARTS.
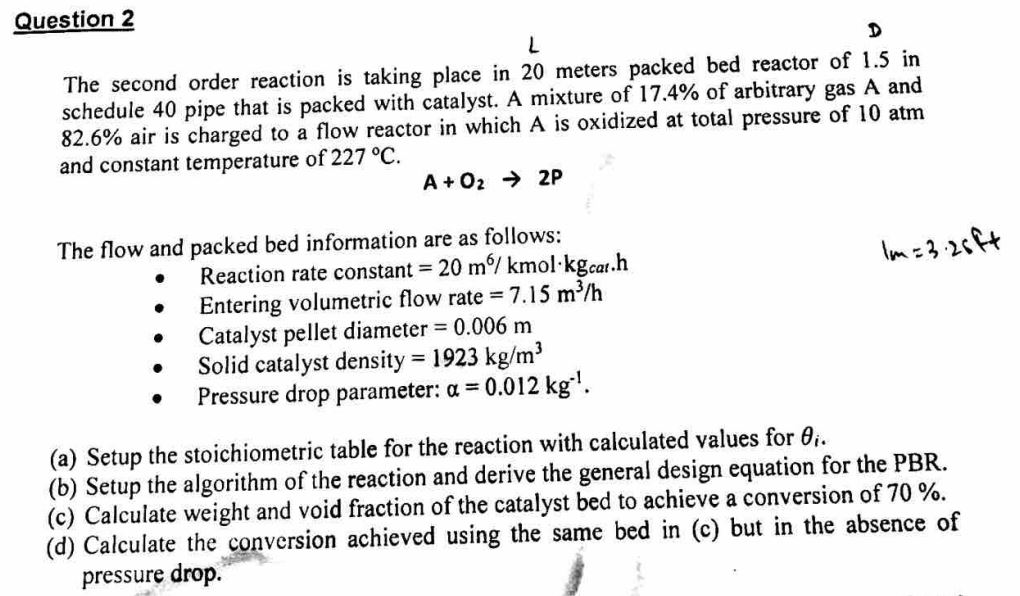
L D The second order reaction is taking place in 20 meters packed bed reactor of 1.5 in schedule 40 pipe that is packed with catalyst. A mixture of 17.4% of arbitrary gas A and 82.6% air is charged to a flow reactor in which A is oxidized at total pressure of 10atm and constant temperature of 227C. A+O22P The flow and packed bed information are as follows: - Reaction rate constant =20m6/kmolkgcath 1m=3.25+4 - Entering volumetric flow rate =7.15m3/h - Catalyst pellet diameter =0.006m - Solid catalyst density =1923kg/m3 - Pressure drop parameter: =0.012kg1. (a) Setup the stoichiometric table for the reaction with calculated values for i. (b) Setup the algorithm of the reaction and derive the general design equation for the PBR. (c) Calculate weight and void fraction of the catalyst bed to achieve a conversion of 70%. (d) Calculate the conversion achieved using the same bed in (c) but in the absence of pressure drop
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


