Question: PLEASE PROVIDE EXPLANATION SO I UNDERSTAND WHAT I AM DOINGWRONG, ETC!!! Determine the direction the following reactions will progress. Assume that the reactants and products
PLEASE PROVIDE EXPLANATION SO I UNDERSTAND WHAT I AM DOINGWRONG, ETC!!!
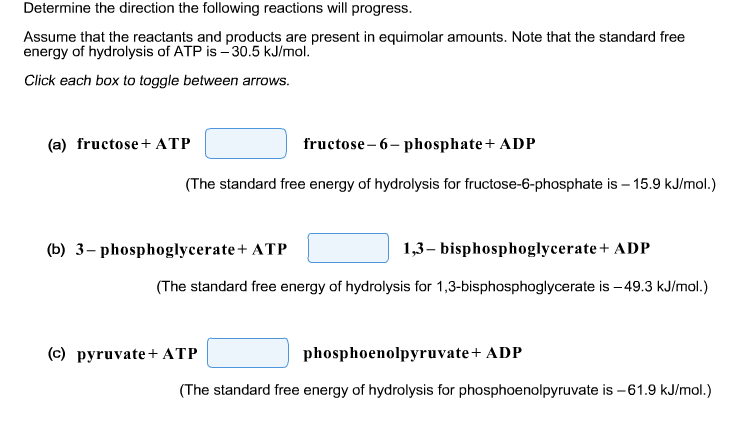
Determine the direction the following reactions will progress. Assume that the reactants and products are present in equimolar amounts. Note that the standard free energy of hydrolysis of ATP is -30.5 kJ/mol. Click each box to toggle between arrows. (a) fructose + ATP fructose-6-phosphate + ADP (The standard free energy of hydrolysis for fructose-6-phosphate is -15.9 kJ/mol.) 1,3-bisphosphoglycerate + ADP (The standard free energy of hydrolysis for 1,3-bisphosphoglycerate is -49.3 kJ/mol.) (b) 3-phosphoglycerate + ATP (c) pyruvate + ATP phosphoenolpyruvate + ADP (The standard free energy of hydrolysis for phosphoenolpyruvate is -61.9 kJ/mol.)
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
a The direction of a chemical reaction is determined by the relative free energy of the reactants and products If the free energy of the products is l... View full answer

Get step-by-step solutions from verified subject matter experts


