Question: Please provide full solution and graph The following are data for the adsorption of CO on wood charcoal at 0C. The following pressure P is
Please provide full solution and graph
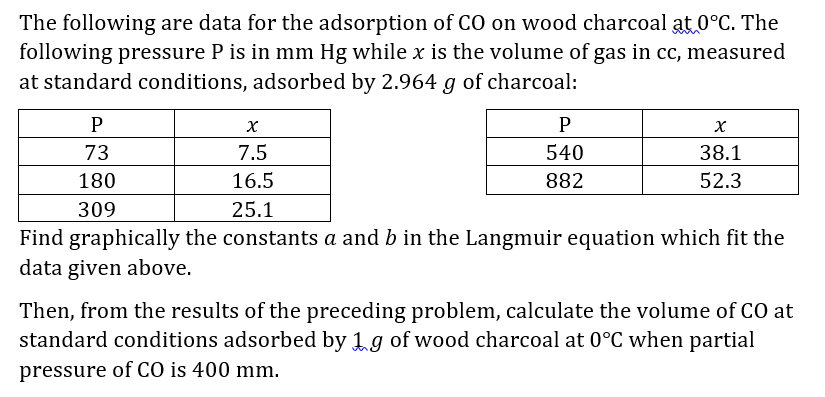
The following are data for the adsorption of CO on wood charcoal at 0C. The following pressure P is in mm Hg while x is the volume of gas in cc, measured at standard conditions, adsorbed by 2.964 g of charcoal: P P 73 7.5 540 38.1 180 16.5 882 52.3 309 25.1 Find graphically the constants a and b in the Langmuir equation which fit the data given above. Then, from the results of the preceding problem, calculate the volume of CO at standard conditions adsorbed by 1 g of wood charcoal at 0C when partial pressure of CO is 400 mm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


