Question: please provide full solution Use electrode potentials from the exam data sheet to calculate K for the following reaction at 25C : Zn(s)+2H+(aq)Zn2+(aq)+H2(g) a) 1.391059
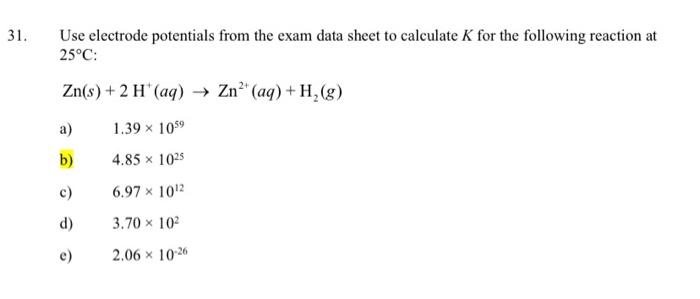

Use electrode potentials from the exam data sheet to calculate K for the following reaction at 25C : Zn(s)+2H+(aq)Zn2+(aq)+H2(g) a) 1.391059 b) 4.851025 c) 6.971012 d) 3.70102 e) 2.061026 ELECTRODE POTENTIALS, E (V)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


