Question: Please provide steps on how to solve this using Dalton's Law. Helium is collected over water at 25.0C and 1.00-atm total pressure. What is the
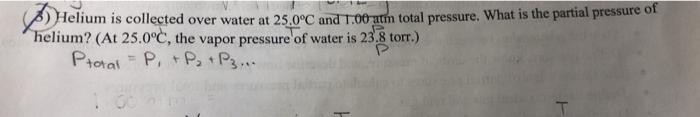
Helium is collected over water at 25.0"C and 1.00-atm total pressure. What is the partial pressure of helium? (At 25.0C, the vapor pressure of water is 23.8 torr.) Ptotal P + P + P3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


