Question: PLEASE READ: Need 4 based on mechanism from 3 PLEASE READ: Need 4 based on mechanism from 3 3. Cumene decomposition is proposed to follow
PLEASE READ: Need 4 based on mechanism from 3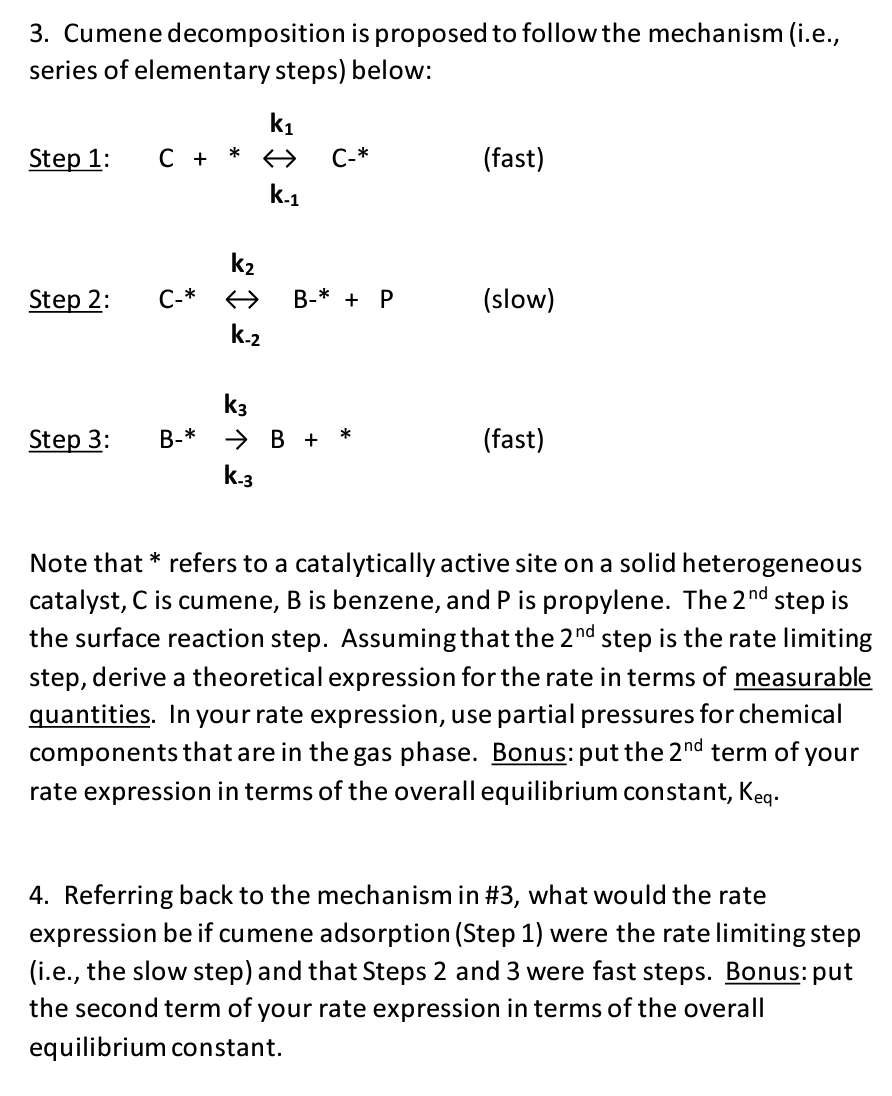 PLEASE READ: Need 4 based on mechanism from 3
PLEASE READ: Need 4 based on mechanism from 3
3. Cumene decomposition is proposed to follow the mechanism (i.e., series of elementary steps) below: Step 1: C + * ki k-1 C-* (fast) Step 2: C-* k2 > k-2 B-* + P (slow) Step 3: B-* k3 B + * k-3 (fast) Note that * refers to a catalytically active site on a solid heterogeneous catalyst, C is cumene, B is benzene, and P is propylene. The 2nd step is the surface reaction step. Assuming that the 2nd step is the rate limiting step, derive a theoretical expression for the rate in terms of measurable quantities. In your rate expression, use partial pressures for chemical components that are in the gas phase. Bonus: put the 2nd term of your rate expression in terms of the overall equilibrium constant, Keq. 4. Referring back to the mechanism in #3, what would the rate expression be if cumene adsorption (Step 1) were the rate limiting step (i.e., the slow step) and that Steps 2 and 3 were fast steps. Bonus: put the second term of your rate expression in terms of the overall equilibrium constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


