Question: please select all the answer that may apply Atomic orbitals such as s,p,d, and f have specitic shapes and orientations in an atom. The hybridization
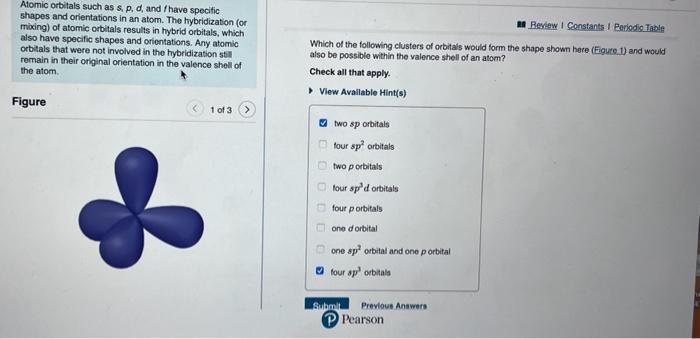

Atomic orbitals such as s,p,d, and f have specitic shapes and orientations in an atom. The hybridization (or mixing) of atomic orbitals results in hybrid orbitals, which also have specific shapes and orientations. Any atomic orbitals that were not involved in the hybridization stil remain in their original orientation in the valence shell of the atom. Which of the following clusters of orbitals would form the shape shown here (Figure.1) and would also be possible within the valence shell of an atom? Check all that apply. Figure View Avallable Hint(s) two sp orbitals four sp2 orbitals two porbitals lour sp3d orbitals four porbitals ono derbital one sp2 orbital and one p orbital four sp3 orbials Alomic orbitals such as s, p,d, and f have specilic shapes and orientations in an atom. The hybridization (or maxing) of atomic orbitals results in hybrid orbitals, which also have specific shapes and orientations. Any atomic orbitals that were not involved in the hybridization still Which of the following clusters of orbitals could form a shape similar to that shown here (Eigure 2 ) in remain in their original orientation in the valence shell of the atom. the valence sholl of an isolated atom or one about to enter into bonding with other atoms? Chock all that apply. View Available Hint(s) Figure one dorbital five dorbitals five sp3 orbitals five sp3d orbitals five sp3d2 orbitals two sp orbitals and three sp2 orbitals three sp2 orbitals and two orbitals three sp2 orbitals and one p orbital
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


