Question: please send me as soon as possible! Exercise 16 The adsorption process using Powder Activated Carbons (PACS) is used to reduce the concentration of the
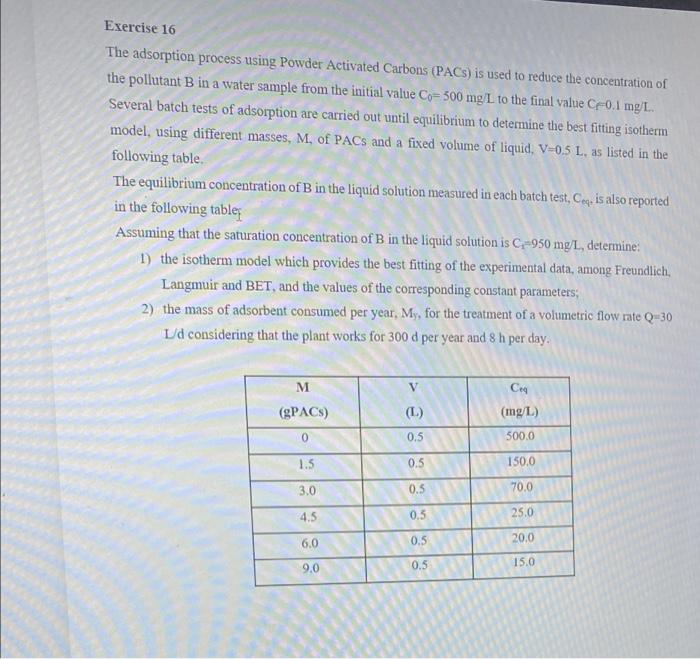
Exercise 16 The adsorption process using Powder Activated Carbons (PACS) is used to reduce the concentration of the pollutant B in a water sample from the initial value Co=500 mg/L to the final value C0.1 mg/L. Several batch tests of adsorption are carried out until equilibrium to determine the best fitting isotherm model, using different masses, M. of PACs and a fixed volume of liquid, V=0.5 L, as listed in the following table The equilibrium concentration of B in the liquid solution measured in each batch test, Ceq is also reported in the following tablet Assuming that the saturation concentration of B in the liquid solution is C-950 mg/L, determine: 1) the isotherm model which provides the best fitting of the experimental data, among Freundlich, Langmuir and BET, and the values of the corresponding constant parameters, 2) the mass of adsorbent consumed per year, My, for the treatment of a volumetric flow rate Q=30 1/d considering that the plant works for 300 d per year and 8 h per day. M V Ca (mg/L) (PACS) (L.) 0 0.5 500.0 1.5 0.5 150,0 3.0 0.5 70.0 4.5 0.5 25.0 6.0 0.5 20.0 9.0 0.5 15.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


