Question: Please show a complete solution. A single vapor-liquid equilibrium point for the water (1) + ethanol (2) system is experimentally measured at 30C. The experiment
Please show a complete solution.
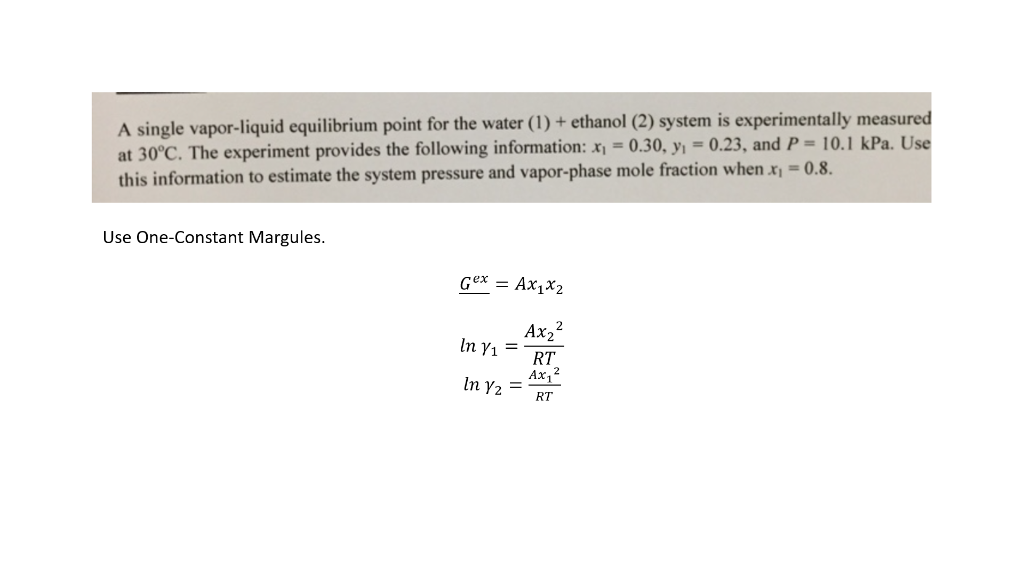
A single vapor-liquid equilibrium point for the water (1) + ethanol (2) system is experimentally measured at 30C. The experiment provides the following information: x1=0.30,y1=0.23, and P=10.1kPa. Use this information to estimate the system pressure and vapor-phase mole fraction when x1=0.8. Use One-Constant Margules. Gex=Ax1x2ln1=RTAx22ln2=RTAx12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


