Question: Please show all the process and steps thanks 1) A water stream contaminated by benzene is to be steam stripped. The goal of this process
 Please show all the process and steps thanks
Please show all the process and steps thanks
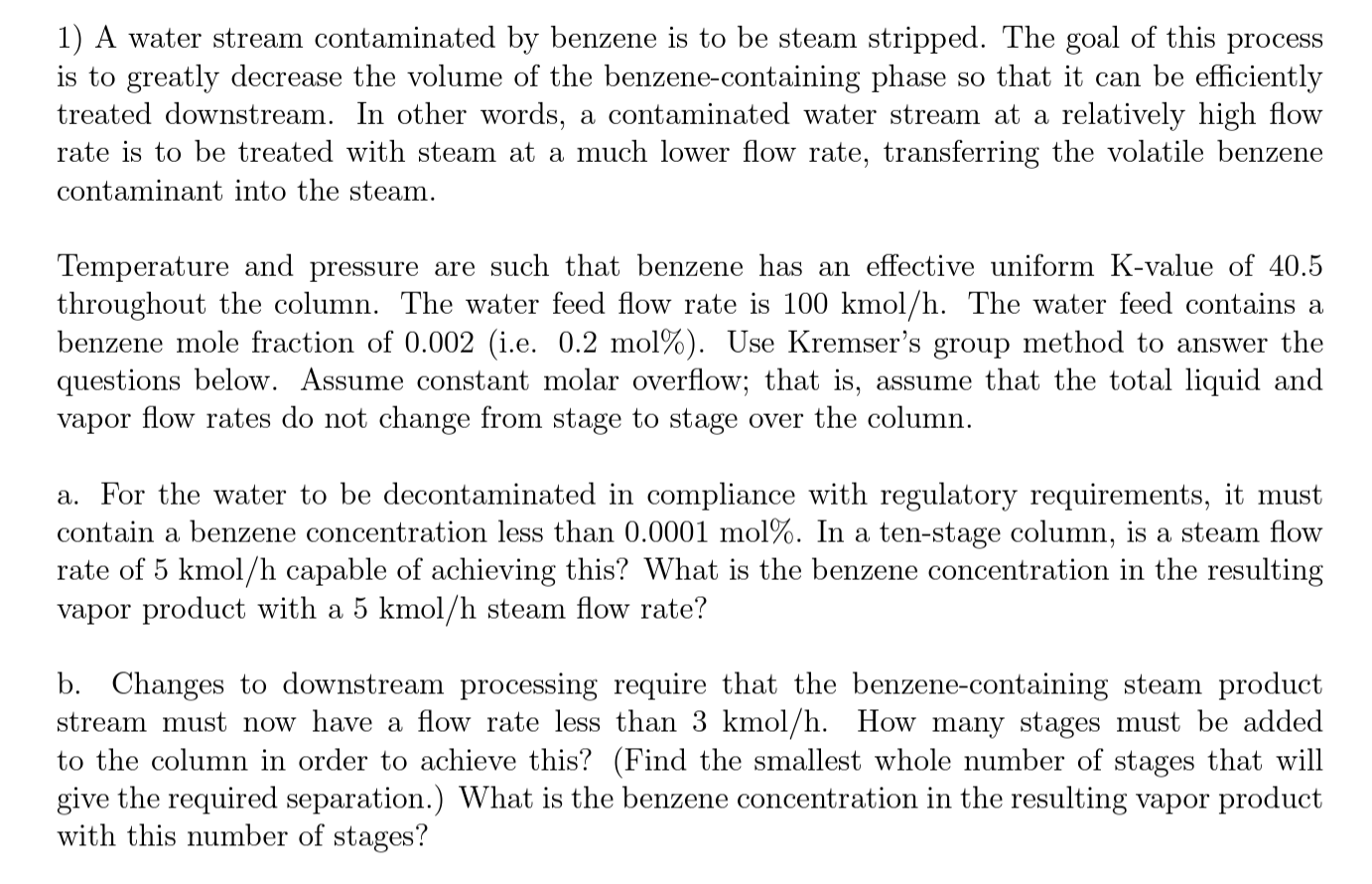
1) A water stream contaminated by benzene is to be steam stripped. The goal of this process is to greatly decrease the volume of the benzene-containing phase so that it can be efficiently treated downstream. In other words, a contaminated water stream at a relatively high flow rate is to be treated with steam at a much lower flow rate, transferring the volatile benzene contaminant into the steam. Temperature and pressure are such that benzene has an effective uniform K-value of 40.5 throughout the column. The water feed flow rate is 100kmol/h. The water feed contains a benzene mole fraction of 0.002 (i.e. 0.2mol% ). Use Kremser's group method to answer the questions below. Assume constant molar overflow; that is, assume that the total liquid and vapor flow rates do not change from stage to stage over the column. a. For the water to be decontaminated in compliance with regulatory requirements, it must contain a benzene concentration less than 0.0001mol%. In a ten-stage column, is a steam flow rate of 5kmol/h capable of achieving this? What is the benzene concentration in the resulting vapor product with a 5kmol/h steam flow rate? b. Changes to downstream processing require that the benzene-containing steam product stream must now have a flow rate less than 3kmol/h. How many stages must be added to the column in order to achieve this? (Find the smallest whole number of stages that will give the required separation.) What is the benzene concentration in the resulting vapor product with this number of stages
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


