Question: please show all work and full stoichiometric table please 1. An elementary, reversible gas phase reaction occurs in an adiabatic packed bed reactor: A +
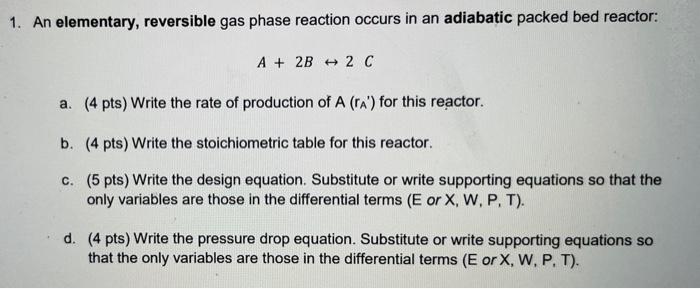
1. An elementary, reversible gas phase reaction occurs in an adiabatic packed bed reactor: A + 2B + 2C a. (4 pts) Write the rate of production of A (A) for this reactor. b. (4 pts) Write the stoichiometric table for this reactor. C. (5 pts) Write the design equation. Substitute or write supporting equations so that the only variables are those in the differential terms (E or X, W, P, T). d. (4 pts) Write the pressure drop equation. Substitute or write supporting equations so that the only variables are those in the differential terms (E or X, W, P. T). 1. An elementary, reversible gas phase reaction occurs in an adiabatic packed bed reactor: A + 2B + 2C a. (4 pts) Write the rate of production of A (A) for this reactor. b. (4 pts) Write the stoichiometric table for this reactor. C. (5 pts) Write the design equation. Substitute or write supporting equations so that the only variables are those in the differential terms (E or X, W, P, T). d. (4 pts) Write the pressure drop equation. Substitute or write supporting equations so that the only variables are those in the differential terms (E or X, W, P. T)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


